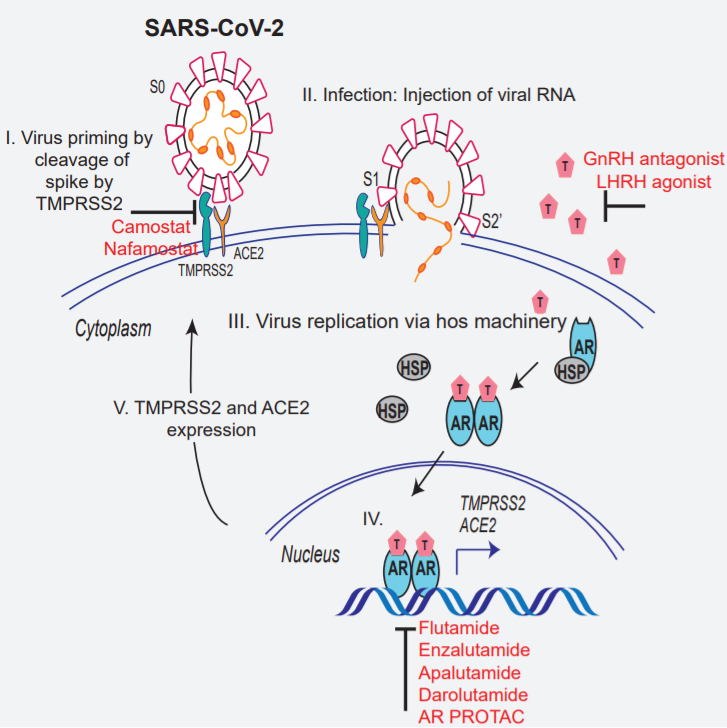
Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19
this is the re-uploaded version of my previous Naver Blog Posting at 2022-02-25
Deng, Q., ur Rasool, R., Russell, R. M., Natesan, R., & Asangani, I. A. (2021). Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19. IScience, 24(3). text
Highlights
- Androgen regulates the expression of SARS-CoV-2 receptor ACE2 and TMPRSS2
- TMPRSS2 interacts with ACE2 in prostate and lung cells
- Camostat blocks TMPRSS2-mediated cleavage of SARS-CoV-2 Spike
- Androgen deprivation or AR antagonists attenuate SARS-CoV-2 Spike-mediated cell entry
Summary
- evidence for the transcriptional regulation of SARS-CoV-2 host cell receptor ACE2 and TMPRSS2 by androgen in mouse and human cells
- endogenous interaction between TMPRSS2 and ACE2 in human cells and validate ACE2 as a TMPRSS2 substrate
- camostat(TMPRSS2 inhibitor) blocks the cleavage of pseudotype SARS-CoV-2 surface Spike without disrupting TMPRSS2-ACE2 interaction
- androgen-deprivation, anti-androgens, or camostat attenuated the SARS-CoV-2 S-mediated cellular entry
⇒clinical evaluations of TMPRSS2 inhibitors and androgen-deprivation therapy/androgen receptor antagonists alone or in combination with antiviral drugs as early as clinically possible to prevent COVID-19 progression.
Introduction
increased morbidity and mortality in men suggests a potential role for androgen in infection and host response.
host immune response to SARS-CoV-2 ⇒ cytokine strom involving the upregulation of TNF-α(tumor necrosis factor-α), IL-1β, IL-6, MCP-1(monocyte chemoattractant protein-1), MIP1α(macrophage inflammatory protein 1-α)
reasonable to hypothesize that the inflammatory signaling accompanying severe COVID-19 disease could cause cancer progression
⇒early intervention of COVID-19 management should be considered in patients with cancer who are more susceptible to SARS-CoV-2 infection due to their immunosuppressive state
virus’ entry: mediated by trimers of the transmembrane spike glycoprotein(S)
S trimer: binds to ACE2(angiotensin-converting enzyme 2) and mediated subsequent viral uptake and fusion dependent on processing by host proteases.
TMPRSS2, cathepsin B and L, furin etc.. several host protease: suggested to promote the entry.
relevant target cell, cleavage of S by TMPRSS2 activates the ptn for membrane fusion via extensive, irreversible conformational changes.
TMPRSS2: androgen-regulated gene associated with prostate cancer
contributes to prostate cancer pathogenesis by aberrantly driving oncogenic transcription
(somatic gene rearrangement results in oncogenic ERG expression under androgen receptor signaling. Erg is Tmprss2’s promoter/enhancer)
TMPRSS2: promote metastatic spread of prostate cancer through HGF activation.
ACE2: zinc-dependent metalloprotease - high expression at heart, testis, kidney
In this study
- evidence for the AR-mediated transcriptional regulation of TMPRSS2 and ACE2 in mice and human prostate and lung cells.
- androgen deprivation in mice by castration, or anti-androgen treatment in vitro led to a reduction in TMPRSS2 and ACE2 transcript and protein levels.
- TMPRSS2 and ACE2: interact in cis in prostate and lung cells, and the inhibition of TMPRSS2 protease activity by camostat blocked Spike priming
- androgen deprivation, anti-androgens, or camostat treatment attenuated the SARS-CoV-2 pseudotype entry in lung and prostate cells ⇒ these drug combinations could be effective in attenuating COVID-19 disease progression in men with or without prostate cancer.
RESULTS
Systemic androgen deprivation affects TMPRSS2 and ACE2 expression in mice
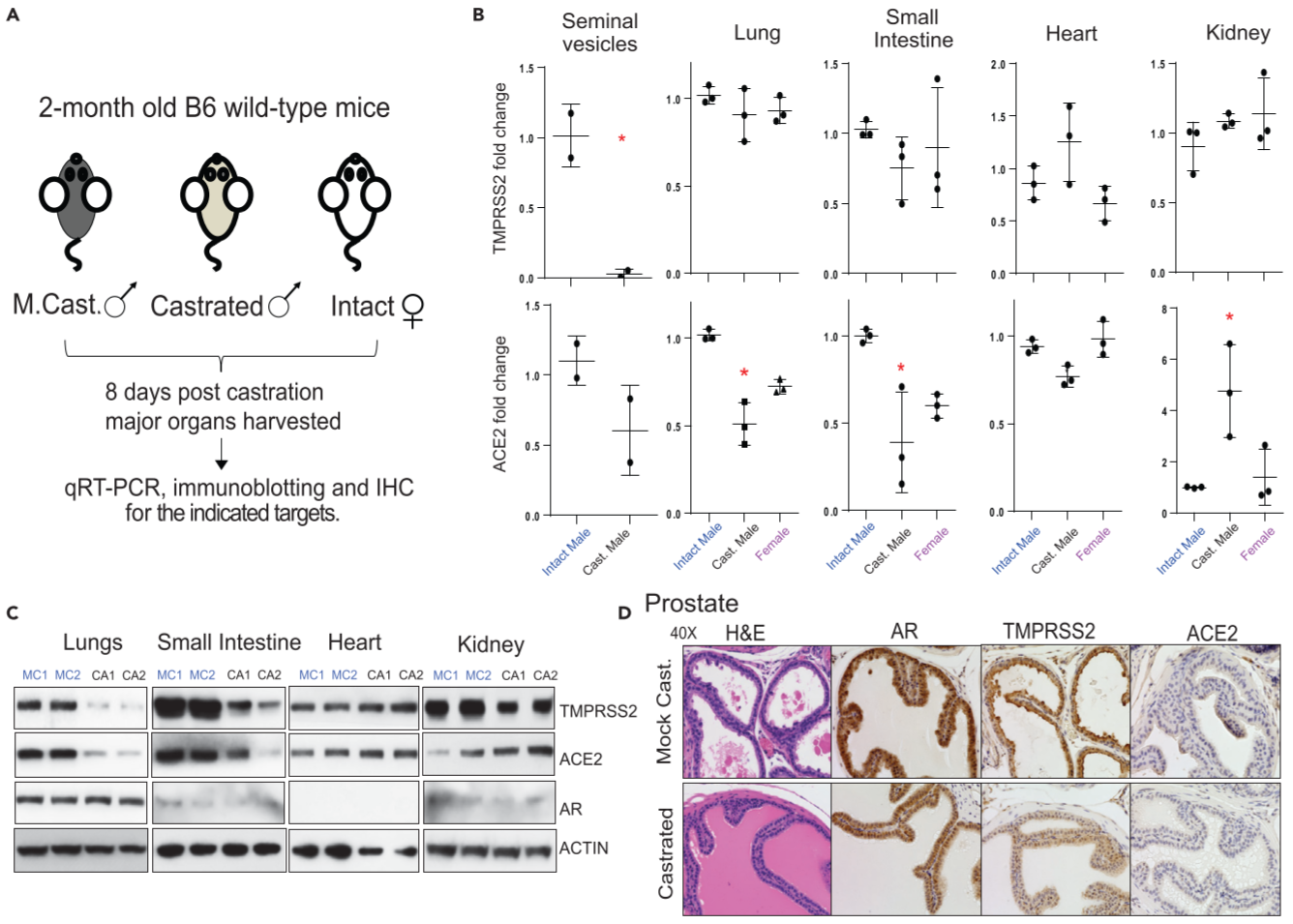
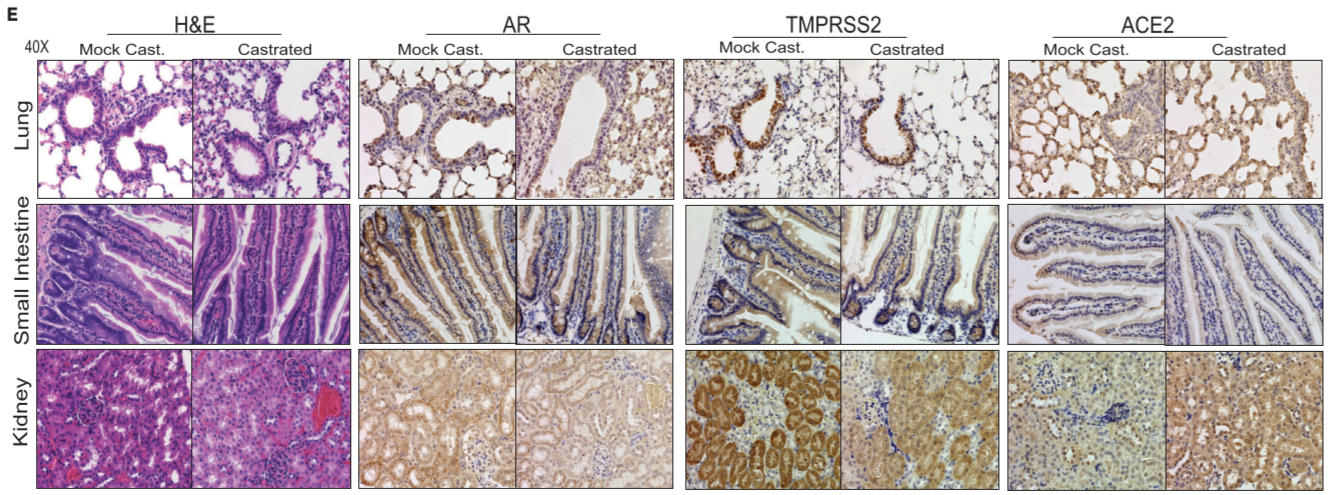
Figure 1. Effect of androgen deprivation by castration on the expression of TMPRSS2 and ACE2 in adult male mice
(A) Schematic depicting the castration experiment in 8- to 9-week-old wild-type C57BL/6J mice
(B) qRT-PCR analysis for TMPRSS2 and ACE2 ⇒ highly hormone-responsive seminal vesicles from the male mice served as a positive control for the effect of androgen deprivation on the target genes
(C) Immunoblot analysis
(D) Immunohistochemistry analysis in the prostate ⇒ reduced TMPRSS2 staining in the castrated group and lack of/sparce ACE2 staining in both groups
(E) Immunohistochemistry analysis in all the tested organs ⇒ reduced TMPRSS2 staining in the bronchial epithelium of lung, columnar epithelium of small intenstine, and proximal convoluted tubules in the kidney, in the castrated group. reduced ACE2 staining in alveolar epithelium of the lungs and columnar epithelium of small intestine and increased staining in the kidney from the castrated group
androgen deprivation has an effect on the expression of TMPRSS2 and ACE2, particularly in the lung, small intestine, and kidney. Although there was a discrepancy concering transcrip and protein levels of TMPRSS2 in the tested tissues, post-transcriptional regulation of TMPRSS2 by androgen-regulated microRNA could not be ruled out.
AR regulates TMPRSS2 and ACE2 expression in human prostate and lung cells
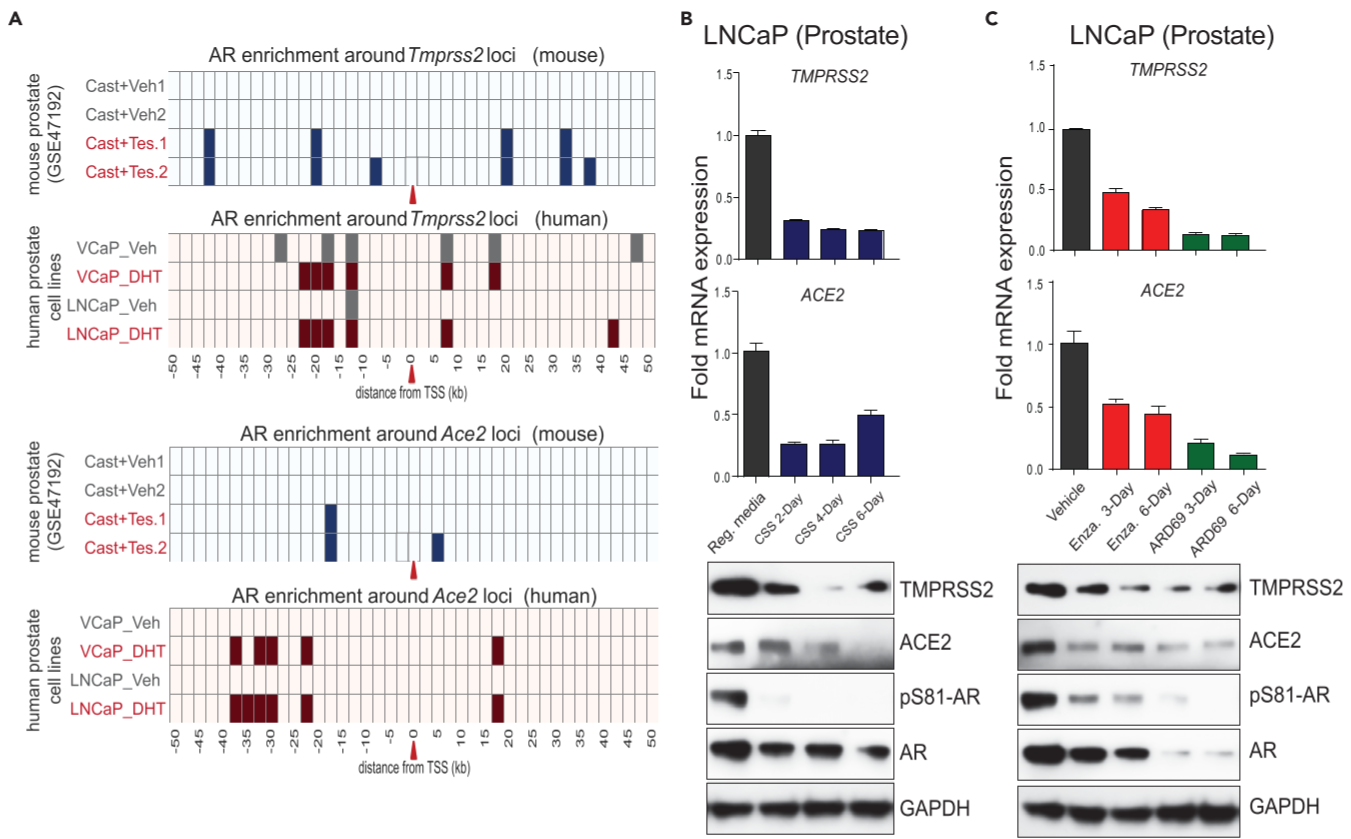
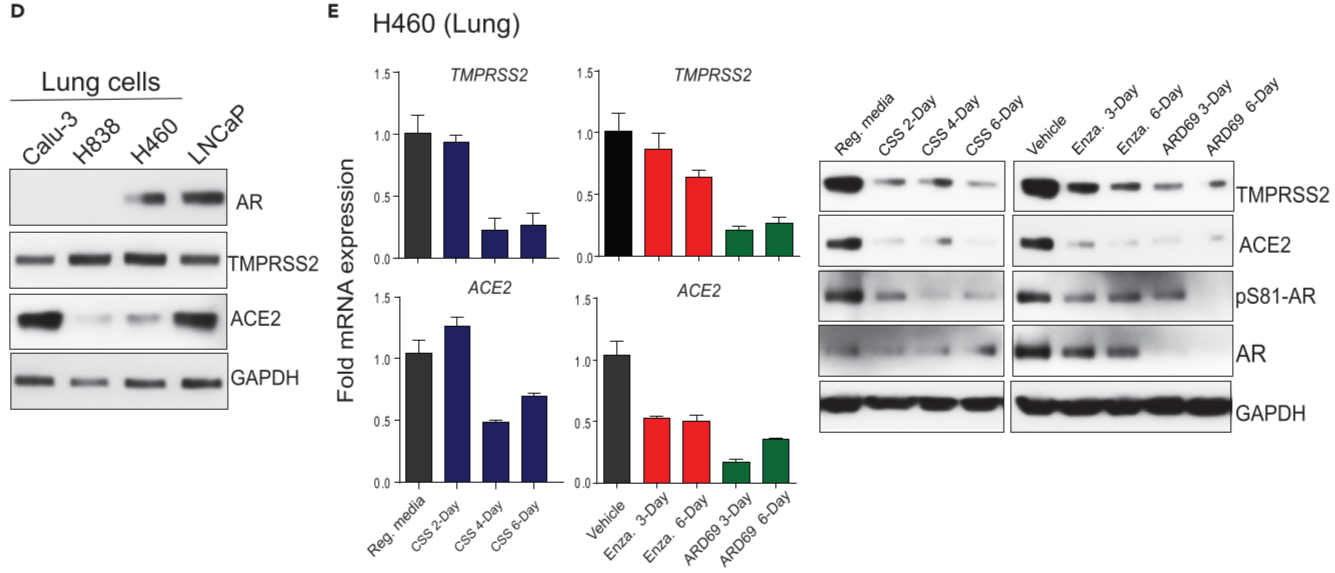
Figure 2. AR-mediated transcriptional regulation of TMPRSS2 and ACE2 in human prostate and lung cells
(A) AR chromatin immunoprecipitation (ChIP)-seq data for AR-binding sites within 50kb of the transcription start sites(TSSs) of the Tmprss2 and Ace2 genes in mouse prostate ⇒ multiple AR peaks were observed in the upstream of the Tmprss2 TSS in the testosterone-treated prostate, DHT-treated human prostate cell lines.
(B-C) androgen deprivation, anti-androgen, or AR degradation results in the loss of TMPRSS2 and ACE2 expression. above: qRT-PCR analysis, below: immunoblot analysis for the indicated protein :: Reg. media: regular media with 10% serum, CSS: charcoal striped serum, Enza: Enzalutamide at 25μM, ARD69: AR degrader at 250nM
(D) Immunoblot showing the expression of AR, TMPRSS2, ACE2, GAPDH in a panel of human lung cell lines; LNCaP - positive control
(E) qRT-PCR & immunoblot analysis as in (B), (C) for human lung cell line H460
potential role of AR in regulating SARS-CoV-2 receptor and co-receptor in prostate and lung cells.
TMPRSS2 interacts with ACE2 in prostate and lung cells
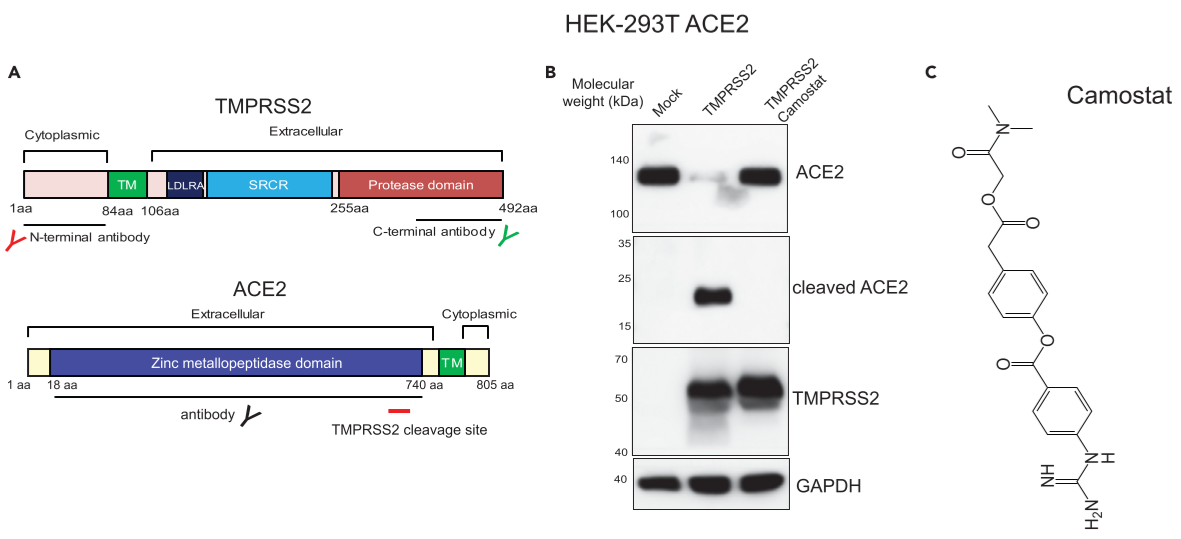
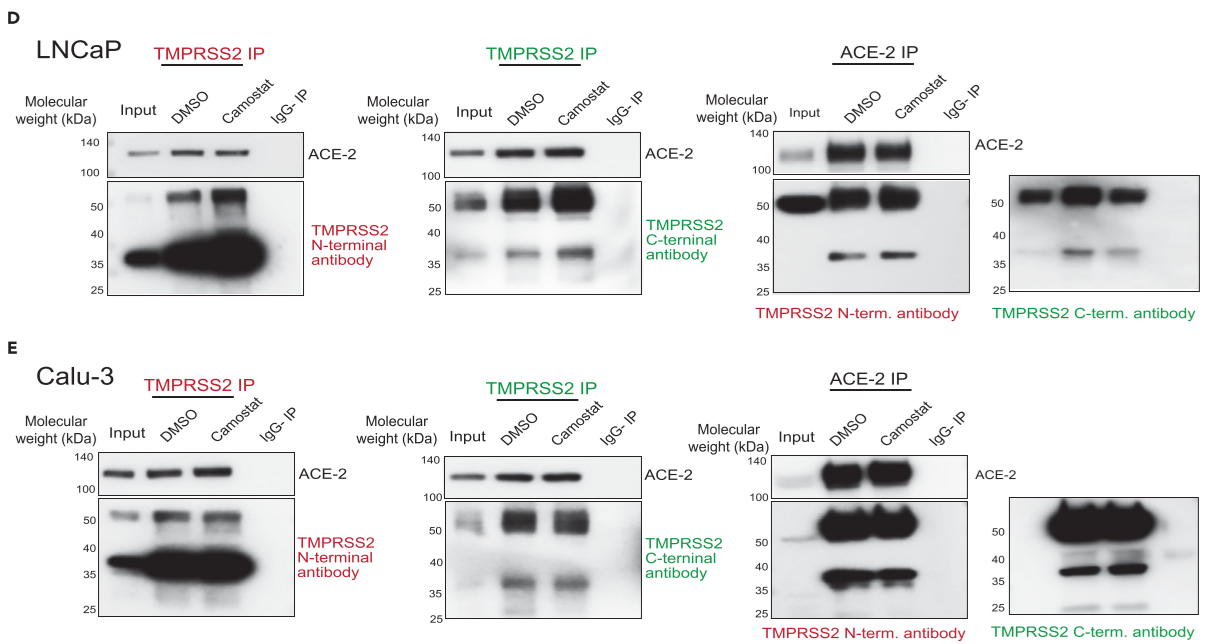
Figure 3. TMPRSS2 physically interacts with ACE2 in prostate and lung cells
(A) Schematic showing the domain structure of human TMPRSS2 and ACE2 protein.
(B) TMPRSS2 cleaves ACE2. HEK 293T ACE2 cells were transfected with TMPRSS2-encoding plasmid, 6h after; treated with DMSO or camostat
(C) chemical structure of camostat
(D) TMPRSS2 and ACE2 physically interact and are not disrupted by camostat, a TMPRSS2 inhibitor. Reciprocal immunoprecipitation with LNCaP protein extracts, IgG pull down - negative control
(E) same with D, with Calu-3 protein extracts
the treatment of cells with camostat did not reduce the amount of ACE2 being pulled down by TMPRSS2, and vice versa, suggesting the endogenous TMPRSS2-ACE2 complex to be stable and not dependent on proteolytic cleavage. A similar endogeneous ACE2-TMPRSS2 interaction was observed in AR-positive H46 lung cells. ⇒ endogeneous association between TMPRSS2 and ACE2 in prostate and lung cells
TMPRSS2 inhibition blocks S priming without affecting its interaction with ACE2
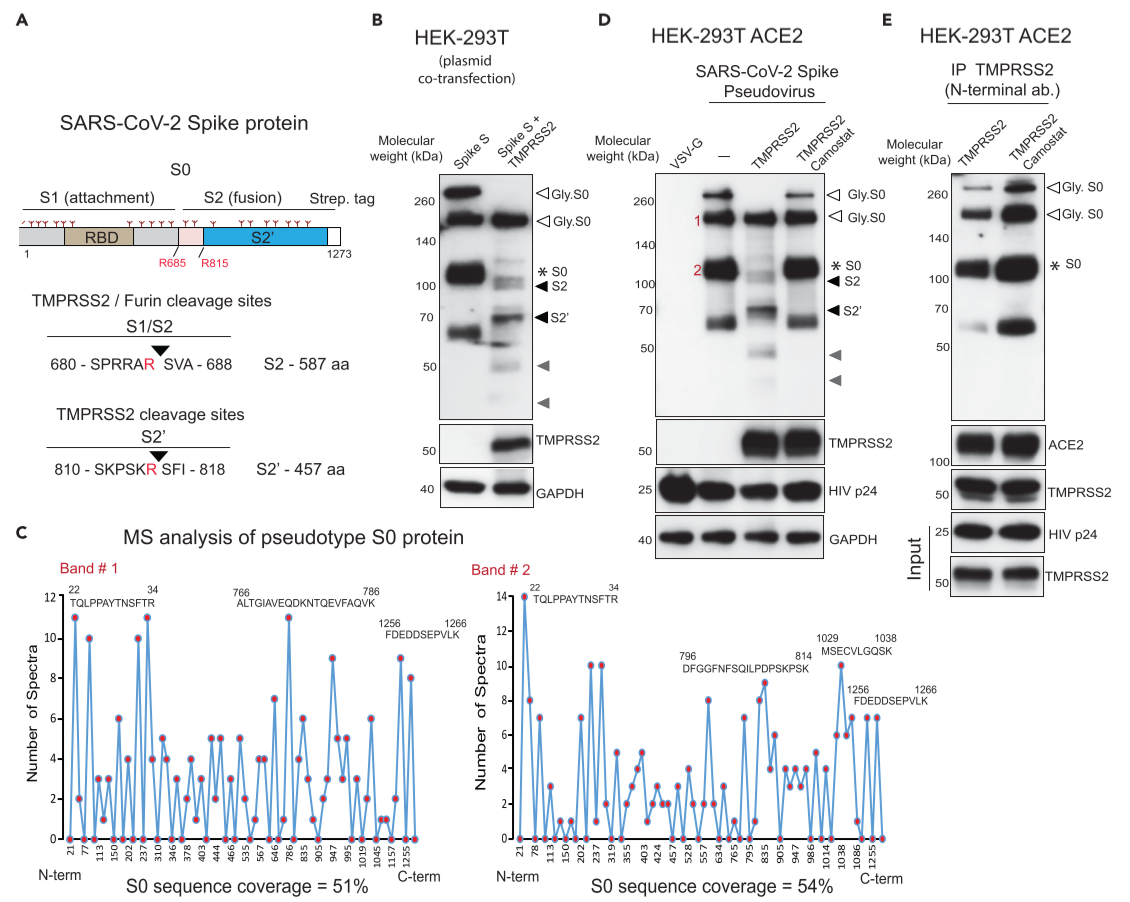
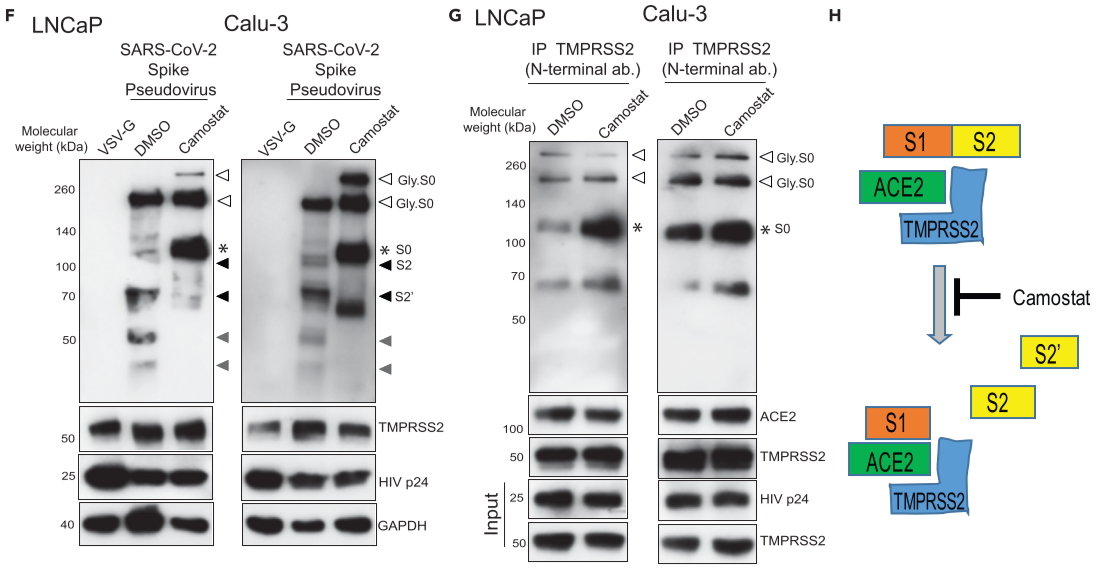
Figure 4. TMPRSS2 in complex with ACE2 cleaves SARS-CoV-2 Spike protein
(A) Schematic representation of the expression construct of full-length SARS-CoV-2 spike(S0) protein that has S1 and S2 segments involved in virus attachment and fusion, respectively. (RBD: receptor-binding domain)
(B) TMPRSS2 cleaves glycosylated SARS-2-S protein. HEK293T cells were transfected with SARS-2-S plasmid with or without TMPRSS2-encoding plasmid. open arrow, *: glycosylated and non-glycosylated uncleaved S0 protein, respectively. black - TMPRSS2-cleaved S2 and S2’, gray - TMPRSS2-cleaved potential S2 and S2’ fragments arising from non-glycosylated S0
(C) Mass spectrometric(MS) analysis of pseudotype-incorporated S0 protein.
(D) HEK293T ACE2-overexpressing cells were transfected with TMPRSS2 plasmid: VSV-G-containing pseudotype virus - negative control, HIVp24 - positive control for viral inoculation: Pseudotype SARS-CoV-2 Spike is cleaved by TMPRSS2, which is blocked by camostat.
(E) Camostat blocks TMPRSS2-mediated cleavage of S0 without disrupting the interaction between TMPRSS2-ACE2-Spike S complex.
(F) Camostat reverses the endogenous TMPRSS2-mediated Spike cleavage in human prostate and lung cells.
(G) Camostat blocks TMPRSS2-mediated cleavage of S0 and S2 without disrupting the interaction between TMPRSS2-ACE2-Spike S complex in LNCaP and Calu-3 cells.
(H) Schematic showing the interaction etween Spike S, ACE2, and TMPRSS2 leading to cleavage of Spike S into S1, S2, and S2’ segments.
clearly demonstrate the crucial role played by TMPRSS2 in SARS-CoV-2 viral fusion to the host cell by Spike protein priming
Camostat alone or in combination with AR-directed therapies reduces SARS-2-S pseudovirus entry into prostate and lung cells
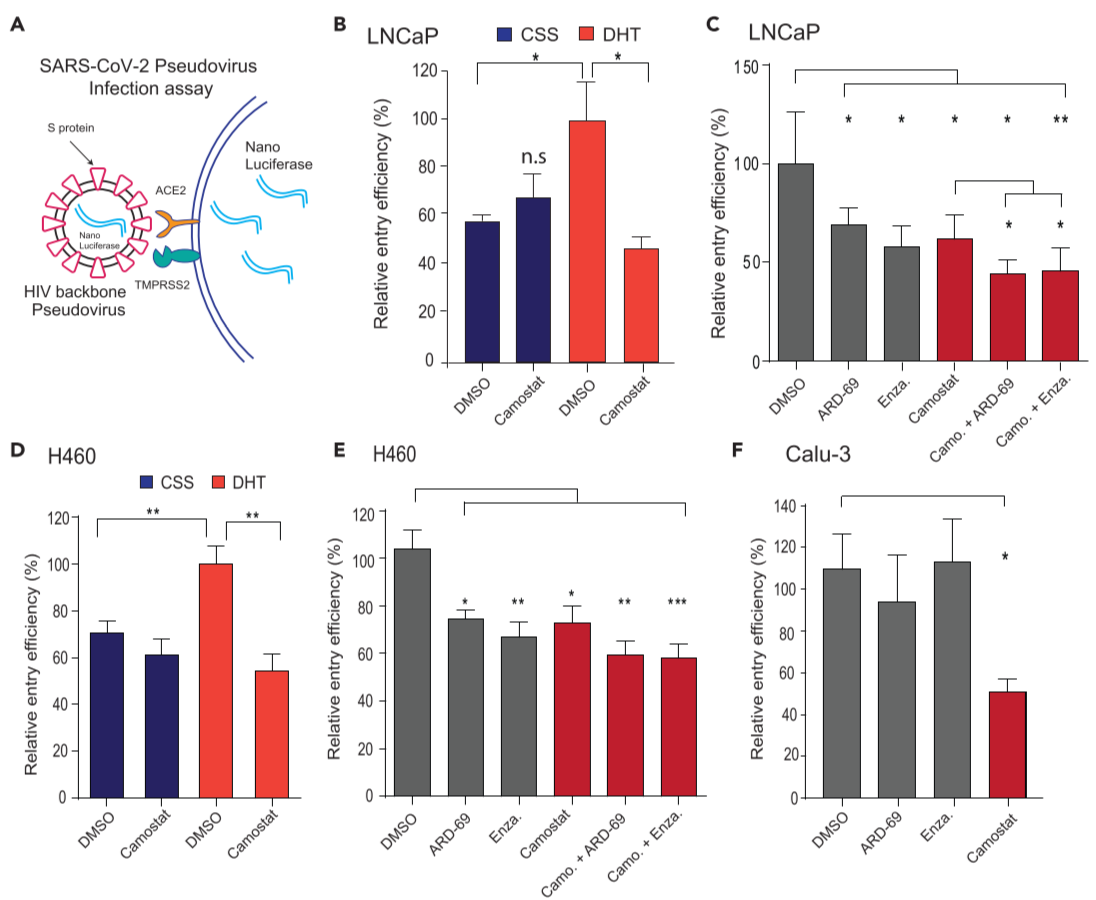
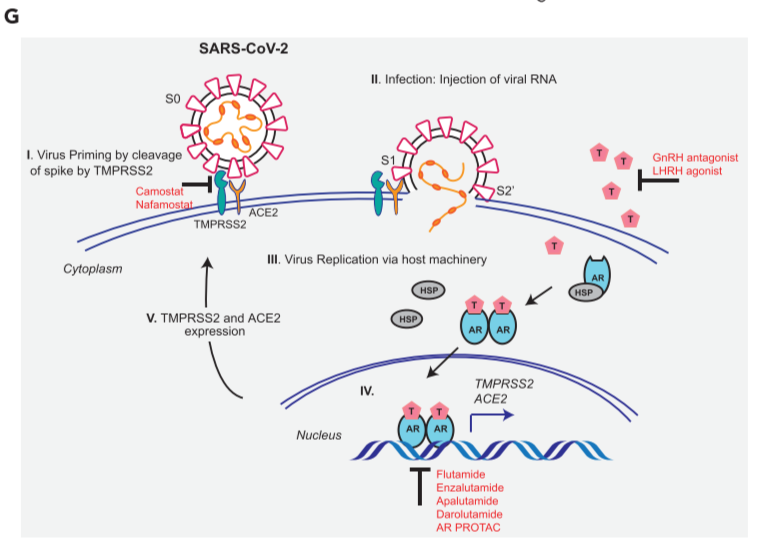
Figure 5. AR-targeted therapies in combination with camostat attenuates the entry of pseudotype SARS-CoV-2 into the host cells
(A) Schematic depicting the SARS-CoV-2 Spike S pseudovirus entry assay. The pseudotype consists of Spike S and nano-luciferase reporter
(B) Androgen deprivation attenuates pseudotype entry.
(C) Anti-androgens or AR degraders with or without camostat attenuate pseudovirus entry.
(D and E) as in (B and C) with AR-positive H460 lung cells.
(F) AR-negative Calu-3 cells do not respond to anti-androgens.
(G) Schematic depicting the role of TMPRSS2 in SARS-CoV-2 Spike cleavage, and androgen-mediated expression of ACE2 and TMPRSS2 that could potentially be targeted by AR-directed therapies.
treatment of AR-positive prostate and lung cells with AR-directed therapies in combination with TMPRSS2 inhibitor efficiently blocks SARS-2-S-mediated viral entry
Discussion
- Implications for developing novel terapeutics targeting the TMPRSS2-ACE2 interface to block Spike activation.
- TMPRSS2 promotes SARS-CoV-2 entry
- ACE2 interaction/cleavage: might promote viral uptake
- SARS-2-S cleavage: activates the S protein for membrane fusion
- Mechanistically, besides androgen, other steroid hormones(estrogen, progesterone, glucocorticoids) may also enhance TMPRSS2 and ACE2 expression in multiple tissues through binding of their respective nuclear receptors to responsive cis elements.
- Androgen: increase circulating neutrophils, increase secretin of IL-10, IL-2, IL-8, TGF-β, decrease the antibody response to viral infections
- and, COVID-19 exhibit increased neutrophils and IL-8
- there were no therapeutic strategies possible for SARS-CoV-2 then, except deamethasone(reduce inflammation), and remdesivir(inhibiting viral replication)
- Our data showing complete block of TMPRSS2-mediated Spike activation by camostat in multiple cell line models provide further credentials for these clinical trials
- It can be possible therapeutic strategy for COVID-19 patients with cancer.
Limitations of the study
Androgen plays a vital role in the human immune response, which we did not address in this article. Research aimed at characterizing the androgen axis in context with SARS-CoV-2 infection will continue in the future.

Leave a comment