Gut microbiome-mediated epigenetic regulation of brain disorder and application of machine learning for multi-omics data analysis
this is the re-uploaded version of my previous Naver Blog Posting at 2022-07-04
Cite: Kaur, H., Singh, Y., Singh, S., & Singh, R. B. (2021). Gut microbiome-mediated epigenetic regulation of brain disorder and application of machine learning for multi-omics data analysis. Genome , 64 (4), 355-371.
Abrstract
- GBA(gut-brain axis)
- connect CNS ↔ ENS(enteric nervous system)
- gut microbiota: key regulator of GBA
- modulate CNS through neuroendocrine and metabolic pathways
- depression, anxiety, autism, stroke, pathophysiology of other neurodegenerative diseases
- microbe-derived metabolites: can influence host metabolism by acting as epigenetic regulators
- ex> butyrate(intestinal bacterial metabolite): histone deacetylase inhibitor → improve learning and memory in animal model
- multi-omics approach that utilizes AI and ML to analyze/integrate omics data is necessary to better understand the role fo the GBA in pathogenesis of neurological disorders
- current understanding of epigenetic regulation of the GBA and proposes a framework to integrate multi-omics data for prediction, prevention, and development of precision health approaches to treat brain disorders
Introduction
CNS↔ENS biochemical link: GBA
ENS: ‘second brain’
- more than 30 neurotransmitters(most identical to CNS): acetylcholine, dopamine, serotonin
- CNS, ENS, ANS(autonomic)
- complex peripheral neural circuit embeded within its wall comprising sensory neurons, motor neurons, and interneurons
- 500 million neurons (5 times larger than spinal cord)
- independently regulate basic gastrointenstinal functions
- brain→ENS indirectly(change in gastrointenstinal motrility, secretion, intenstinal permeability) or directly(signaling molecules)
- gut→brain: neural, endocrine, immune, humoral pathways
- psychiatric disorders(anxiety, depression), neurological, neurodevelopmental disorders(autism, AD, PD..)
- gut microbiota: epigenetic modifications(DNA methylation, histone modifications) → influence brain and behavior
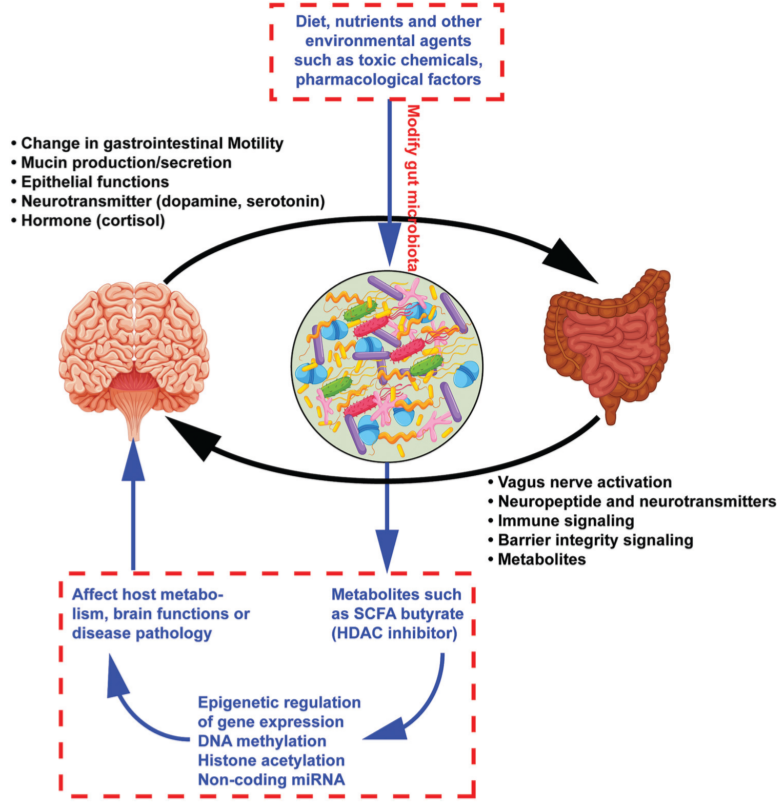
Fig 1. Bidirectional communication between the brain and gut mediated via the gut microbiota: black The effect of gut microbiome on brain functions via epigenetic regulation of gene expression: blue
Gut Microbiome
key regulator of the GBA
total of all microorganisms in gastrointestinal tract
bacteria, fungi, archaea, viruses, protozoans
$10^14$ microbes
symbiotic relationship with gut mucosa ⇒ impart metabolic, immunological, gut protective functions in the host
microbiome: collective genomes of the microorganisms(i.e., microorganism+their set of genes)
- firmicutes, bacteroidetes: 90%
- actinobacteria, proteobacteria: 1~5%
in human diseases…
inflammatory bowel diseases
irritable bowel syndrome
metabolic diseases(obesity, diabetes), allergic diseases, neurodevelopmental illnesses
important in human development, immunity, nutrition
2007 NIH: Human Microbiome Project
provided vast knowledge: dvelopment of reference seq. multi-omics datasets, computational/statistical tools, analytical/clinical protocols → broader research community
Gut microbiota-brain communication (via vagus nerve, neuroendocrine, and neuroimmune pathways)
-
- direct signals to the brain by activating afferent sensory neurons of the vagus nerve
- via neuroimmune and neuroendocrine pathways
- vagus nerve
10th cranial nerve(from brainstem-neck-thorax down to the abdomen)
main component of the parasympathetic NS
crucial bodily functions: control of mood, immune response, digestion, heart rate
modulate gastrointestinal motility, secretion, epithelial permeability
⇒ modify the physical environment that the microbiota inhabit, thus affecting its composition
transmit signals from the gastrointestinal tract to the CNS and can recognize microbioal products or cell wall components
- stimulation of vagus nerve stabilize the abnormal electrical activity in the brain ⇒ treatment of epilepsy and other neurological conditions
ablated gut-related vagal communication → affect adult neurogenesis, stress reactivity, anxiety-and fear-related behavior, cognition (psychiatric disease)
- Gut bacteria’s influence influence the cells of the gastrointestinal tract that produce neurotransmitters and digestive hormones or peptides in the gut ⇒ can alter the brain and behavior diet, environment, probiotics, drugs(antibiotics etc.): affect vagus nerve activity vagal afferent nerve fibers respond to a variety of stimuli(cytokines, nutrients, gut peptides, hormones) vagal receptors: recognize the regulatory gut peptides, inflammatory molecules, dietary components, bacterial metabolites to relay signals to the CNS altered gut microbiota composition/changes ⇒ vagus nerve to alter CNS outputs(active role in mediating neurological functions) this nerve: direct route for gut-to-brain signaling
- Lactobacillus rhamnosus treatment in mice ⇒ reduced stress-induced corticosterone and anxiety- and depression-related behavior(not in vagotomized mice) ⇒vagus: major modulatory constitutive communication pathway bet. bacteria and brain
- Lactobacillus intestinalis, Lactobacillus reuteri cause depression, anhedonia-like phenotypes in antibiotic-treated mice via the vagus nerve
- L.reuteri: target ion channels in enteric sensory neurons ⇒ increased action potential
- Neuroendocrine system
- potential link bet. gut microbiota and neuroendocrine system in various psychiatric, gastrointestinal diseases
- Immunomodulation by the microbiota
- orchestrates microbiota-gut-brain communication
- gut microbiota regulate inflammation, autoimmunity, immune cell trafficking
- contribute to microglial maturation and function
- microglial involvement existing neurological disorders (human) -> altered microbial community composition was observed
- active microbial signaling is required throughout adulthood to preserve microglial maturation
Gut metabolites (short-chain fatty acids)
produce wide range of metabolites
neurotransmitters, hormones, vitamins, short-chain fatty acids(SCFAs)
play an important role in host defense(training host immune system)
affect neuronal functions
- commensal bacteria(anaerobic): fermentation of non-digestible dietary fibers
⇒produce key bacterial metabolites and SCFAs (acetate, propionate, butyrate, valerate)
⇒regulate gut integrity and immune responses and maintain overall host homeostatis
More than 95% of these SCFAs are used as an energy source by colonocytes, and 60-70% of their energy comes from SCFA oxidation.
Role of SCFAs
- act as signaling molecules (involved in systemic lipid metabolism and glucose/insulin regulation)
- cross BBB via monocarboxylate transporters
-
- essential for maintaining the intestinal barrier permeability
- regulate tight junction proteins in the gut
- involve in maintaining BBB permeability
- modulate mammalian cell functions by serving as an energy substrate or by signaling through GPCRs(GPR41, GPR43)
- at Brain, influence CNS immune system (regulating microglial maturation/function) -dysfunction of microglia is involved in the initiation or progression of multiple CNS disease(AD, PD, ASD, depression)
Role of gut microbiota in brain disorders
microbiota dysbiosis in depression, anxiety, autism, neurodegenerative, stroke
can be used as biomarker for the CNS
- brain alters intestinal microenvironment by regulating the gut motility and secretion as well as mucosal immunity via the neuronal glial-epithelial axis and visceral nerves
- gut reacts via changing the bacterial metabolites (neurotransmitters, neuromodulators like SCFAs, gut hormones(leptin, ghrelin etc.))
probiotics/antibiotics usage → behavioral responses
anxiety-like disorders & improvement of anxiety symptoms by regulating intestinal microbiota
Cerebral disorders → various psychiatric symptoms (dementia, mood disorder, stress, anxiety, movement disorder, personality disorder…)
talk therapy + antidepressant medication treatment
antidepressent - directly regulate HPA axis
HPA axis → contribute
Gut microbiota
- development of the HPA axis
- GBA disruption → change intestinal motility/secretion/visceral hypersensitivity → cellular alterations of the entero-endocrine and immune systems
-
ASD
environmental factors
immune system abnormalities
gut microbiota
-
AD
altered gut microbial composition → associated with increased intestinal permeability and increased intestinal inflammation in various models of AD
Gut metabolites as epigenetic regulators
- cell number: 10 times than the totl number of human body cells
- 3~4 million unique genes present (100-150 times more genes in geome)
metabolites: some are absorbed into the systemic circulation and reach distant organs, including brain
some are biologically active, some are further metabolized by host enzymes
-
- important epigenetic regulators: can influence host gene expression
- interact at DNA, RNA, histone levels
DNA methylation, post-transcriptional histone modifications, chromatin restructuring and associated DNA methyltransferases(DNMTs), DNa hydroxylases, histone acetyltransferases, histone deacetylases, histone methyltransferases, histone demethylases
- DNA methylation
CpG regions(C-G rich regions): by DNMTs → suppress gene transcription
diet, neutrients, environmental agents(toxic, pharmacologiclal factors)
no microbiota or no change → long-lasting epigenetic modifications(affect behavior)
- TET: DNA demethylation
- Clostridia etc. dietary fibers to SCFAs, especially butyrate: HDAC inhibitor ⇒enhance chromatin accessibility, activate gene expression
- histone acetylation
- histone methylation
- H3 lysine 4: gene activity increate
- lysine 9: repression
- gut microbiota: alterate chromatin state
- acetate, propionate, folate etc.
- acetate, propionate: HDAC inhibitory activity
- SAM(S-adenosyl methionine) (MAT catlyzes methione to SAM) ⇒DNMT moves methyl group to CpG → gene silence
- 5mC(5-methylcytosine, fifth base of DNA)…

- vagus nerve in epigenetic regulation
- vagus nerve stiulation(VNS) → hippocampal, cortical, blood epigenetic transcriptomes, epigenetically modulates neuronal plasticity, stress-response signaling genes
- glucose affects a cell-specific epigenetic regulation of memory-related genes(Bdnf, Fgf-1)
- glucose-mediated secretion of gut hormones may induce VNS to enhance memory process
- gut microbiota!!
- how gut microbiota influence the epigenetics in brain disorders?(especially neuropsychiatric disorders: anxiety, depression, neurodegenerative)
- bile acids, vitamins, choline metabolites, SCFAs/lipids
- SCFAs: regulate MHC gene expression
- butyrate: HDAC inhibitor
Application of machine learning in multi-omics data analysis

Fig 2. Framework for multi-omics data integration and use of advanced ML approaches to predict the health or diseased state or to find the right therapeutic strategy for an individual person
Discussion
- GBA: biochemical link bet. CNS and ENS ← gut microbiota is the main contributor to variable functions
- gut microbiome ↔ brain function : critical in understanding role of GBA
- gut microbiota → brain
- through neural, endocrine, immune, humoral pathways
- microbiology, epigenetics, computational biology
- metabolites: SCFAs
- host defense, act as signaling molecules involved in lipid metabolism, glucose/insulin regulation
- essential for maintaining intestinal, BBB permeability
- modulate mammalian cell functions by serving as an energy substrate / signaling through GPCR
- (in)directly stimulate vagal nerve → influencing GBA
- epigenetic
- diet, nutrients, environments, probiotics, drugs…
- SCFAs: HDAC inhibitor etc…
- omics/computational biology
- identification of biological factors/mechanisms that connect the onset and progression of neurological diseases to gut microbiome
- disease risk prediction and precision medicine in humans
- but not yet

Leave a comment