WNT signaling in glioblastoma and therapeutic opportunities
this is the re-uploaded version of my previous Naver Blog Posting at 2022-04-30
Lee, Y., Lee, J. K., Ahn, S. H., Lee, J., & Nam, D. H. (2016). WNT signaling in glioblastoma and therapeutic opportunities. Laboratory investigation, 96(2), 137-150. Paper Link
Abstract
- oncogenic roles of WNT signaling in GBM
- current progress in the targeting of WNT signaling as an anti-cancer approach
- genetic and epigenetic alterations that lead to aberrant WNT pathway activation in GBM
- WNT-mediated control of GBM stem cell maintenance and invasion
- cross-talk between WNT and other signaling pathways in GBM
- discovery of agents that can inhibit WNT signaling in preclinical models and the current status of human clinical trials
Main
WNT signaling: development of NSCs(Neural stem cells)←crucial roles
aberrant activation of WNT signaling in NSCs lead to maligant transformation and development of brain tumors
WNT signaling in NSC self-renewal and proliferation
WNT3A: upregulate WNT signaling activity → increase the clonogenic potential of NSCs
activation of β-catenin: increase proliferation of mouse neural progenitor cells(in vivo)
deletion of β-catenin: decreased their proliferation
Glioblatoma(GBM) most common/lethal CNS tumor in adults
surgical resection + chemotherapy(Temozolomide(Temodal): DNA alkylating agent)
genomic studies
- loss-of-function: p53, phosphatase, tensin homolog, neurofibromatosis 1
- hyperactivation: RTK signaling(EGFR(epidermal growth factor receptor), PDGF receptor, MET(hepatocyte GF) receptor etc.)
WNT signaling is aberrantly activated in GBM
promotes GBM growth&invasion
Overview of WNT signaling
critical regulator of cell-cell interactions, cell fate decision, migration
mutation in WNT pathway components ⇒ specific developmental defects
aberrant WNT signaling ⇒ often cancer
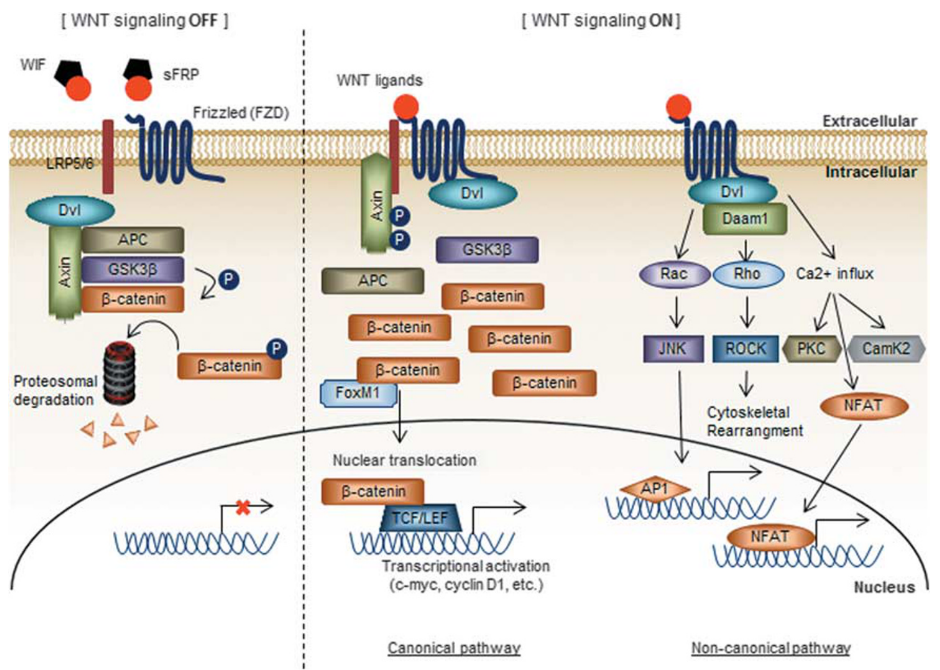
Figure 1. **Overview of WNT signaling pathway
Canonical: β-catenin enters the nucleus, binds to TCF, and transcribes WNT target genes
Non-canonical: β-catenin independent → polarity and cytoskeleton-mediated progress
Canonical WNT Signaling
key regulator in embryonic and adult stem cells
- binding of WNT ligands to cysteine-rich domains of FZD and LRP families(cell surface)
- disassembly of the complex consisting AXIN, APC, GSK3β → stabilizing β-catenin
- β-catenin translocation from cytoplasm to nucleus
- form complex with TCF/LEF → promotes transcription of multiple target genes(c-MYC cyclin D1 etc) (FoxM1 -> nuclear translocation, stabilization promote ← FoxM1 can activate this pathway in a ligand-independent manner!)
Non-Canonical WNT Pathway
affects cell polarity and WNT-Ca$^{2+}$ pathways
contribute to developmental processes (planar cell polarity(Drosophila), convergent extension movements during gastrulation, cell migration of neuronal/epithelial origin)
- Binding of WNT ligands(WNT4, WNT5A, WNT11) to the FZD receptor
- induces recruitment of Dvl and Daam1
3-1. initiates cascade that activates Rac, Rho GTPases
- mediate asymmetric cytoskeletal organization and polarized cell migration
3-2. calcium signaling
- binding of WNT ligand → recruitment of Dvl in complex with a G-protein
- G-protein-dependent release of calcium
- PKC, CamK2
- stimulate the activation of calcineurin ⇒ accumulation of nuclear factor of activated T cells in the nucleus
Genetic/Epigenetic Alterations of WNT Signaling Components
mutations in WNT signaling components → case of WNT pathway activationin tumors
- colorectal tumors / colon cancer
- 85% APC mutation, of the remaining 50% β-catenin mutation
- most APC mutations: loss-of-function
- medulloblastoma
- also WNT components mutation
- β-catenin mutations in exon 3(phosphorylation site) → 18~22%
- additional 5%: mutations in APC or AXIN1
hepatocellular carcinoma/medullobalstoma β-catenin mutation: disruption of phosphorylation/degradation of β-catenin → hyperactivation of WNT signaling
- GBM
- no genomic mutations in β-catenin and APC
- homozygous deletion of FAT1(negative effector of WNT signaling)
- 20%: copy number loss of FAT1
- FAT1 loss is a critical molecular event for WNT activation
- Epigenetic silencing of negative effectors of WNT pathways
- FRPs: bind to WNT and interfere with
- DKK: antagonist of signaling via binding to its co-receptor LRP
- epigenetic silencing of WNT pathway inhibitor genes: frequent in gliomas
- promoter hypermethylation of sFRPs(sFRP1~5), Dickkopf(DKK1, DKK3), Naked(NKD1, NKD2)
- primary GBM 40%: sFRP1, sFRP2, NKD2 promoter hypermethylation
- sFRP: contributes to proliferation/migration of glioma cell
- ectopic expression of sFRP: reduce glioma cell motility (decrease MMP2)
- secondary GBM 50%: DKK1 promoter hypermethylation
Conclusion! epigenetic alteration of WNT signaling components, rather than genomic mutation, plays major role in WNT activationin GBM
WNT Signaling in GBM stemness
WNT Signaling in Stem Cells
Cancer stem cells(CSCs) model
- inhibition of WNT signaling via modulation of primary GBM 40%, LEF and TCF impeded te clonogenic growth of various cancer cells
- WIF1(WNT inhibitory factor 1) induced cellular senescence → impeding stemness & tumor growth
WNT Signaling in GBM stem Cells (GSCs)
GSCs: critical cell population that contributes to GBM malignancy, therapeutic resistance to standard therapies, and recurrence
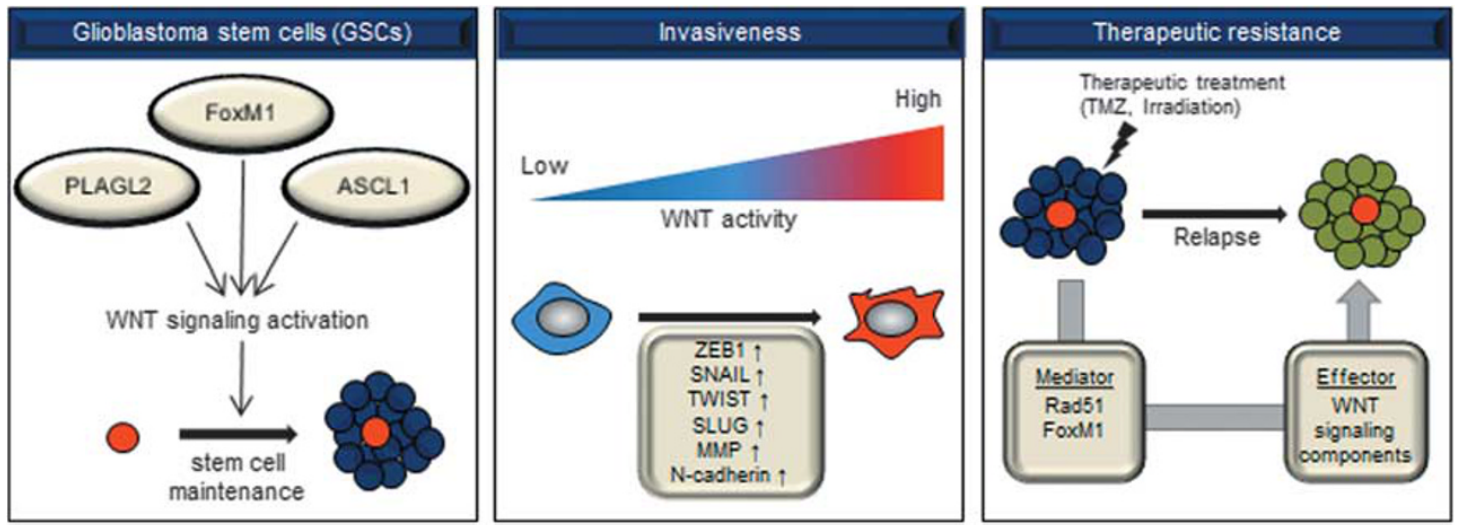
Figure 2. The role of WNT signaling in GBM (1) maintenance of glioblastoma stem cells (2) enhancement migration and invasion (3) induction of multi-drug resistance
-
Depinho group
PLAGL2(chromosome 20q11.21) is amplified in primary GBM specimens & GBM cell lines
maintain self-renewal ability of GBM cells & restrain NSC differentiation
PLAGL2 overexpression in astrocyte / GBM cells → upregulation of WNT signaling components(WNT6, FZD9, FZD2)
PLAGL2: important for stem cell maintenance and gliomagenesis via activation of canonical WNT signaling
-
involvement fo WNT signaling in GSCs via FoxM1
FoxM1 pormotes β-catenin nuclear translocation(directly binding)
expression level of nuclear FoxM1 correlated with that of nuclear β-catenin in GBM patient specimens
high levels of FoxM1 in GSCs → FoxM1 was phosphorylated by MELF(GSC-enriched kinase), promote self-renewal and chemo-resistance of GBM cells
FoxM1 selectively bind to the promoter of Sox2(master regulator of GSC self-renewal, promotes stem cell transcription programs in GSCs)
-
Rheinbay et al : comparative analysis of chromatin state in GSCs with entire tumor → identified a set of developmental TFs unique to GSCs
ASCL1 activates WNT signaling in GSCs ← repressing the negative regulator DKK1
-
Bartscherer et al
Evi(seven-pass transmembrane protein) is involved in the secretion of WNT ligands in Drosophila and human cells → affect both canonical&non-canonical WNT pathways
strongly expressed in gliomas → Evi depletion in glioma cell lines impeded cellular proliferation, clonogenic growth, invasion
-
etc.
Frizzled, Dvl2 etc. overexpressed in GBM → promoted clonogenic growth & stem-like characteristics of GBM cells
WNT Signaling in GBM Invasion
WNT signaling is involved in both tumor invasion and EMT(Epithelial-mesenchymal transition)
WNT signaling activation enhance the motility of bladder, breast, pancreatic cancer cells
overexpression of positive WNT signaling regulators: increase the expression of EMT-associated genes(ZEB1, SNAIL, TWIST, SLUG, N-cadherin)
ectopic expression of a constitutively active β-catenin→induced expression of ZEB1 in GBM cells: increased cell motility
inhibition of β-catenin suppressed cellular invasion in U87MG, LN229 GBM cells
WNT5A: induce migration in GBM cells by activating β-catenin-independent pathway
WNT5A knockdown in glioma cells: significantly inhibited the migratory capacity of these cells without affecting proliferation kinetics
expression of recombinant WNT5A stimulated migration via increase of MMP2 activity
WNT2, FZD2 also are similar
GBM rarely metastasizes to other, but disseminate widely into the neighboring brain parenchyma
almost impossible to perform radical, maximal tumor resection
highly invasive GBM cell population - serial in vivo transplantation assay & mRNA expression profiles analysis
FZ4(positive WNT regulator): causative effector for invasive phenotypes of GBM cells
WNT signaling has critical roles in GBM invasion / provide rationale for targeting WNT signaling as a potentially effective anti-GBM therapeutic approach
WNT Signaling in Therapeutic Resistance
activation of WNT signaling induces drug resistnace in various cancers(ovarian, colon, pancreatic cancer
-
WNT5A was upregulated in oxaliplatin-resistant ovarian carcinoma cell line
ectopic expression of WNT5A conferred greater resistance of ovarian cancer cells to paclitaxel, 5-fluorouracil, epirubicin, etoposide
WNT5A activated Akt signaling and rendered colon cells resistant to histone deacetylase inhibitors
inhibition of WNT5A → increased drug-induced apoptosis in pancreatic cancer
-
In GBM
WNT signaling promotes resistance to temozolomide(standard chemotherapeutic agent for GBM)
FZD2 etc. WNT signaling component activation → temozolomide-resistant subclones
WNT signaling contributes to radioresistance of cancer cells
breast cell model에서 stabilized β-catenin selectively reinforced mammosphere formation and enhancedradioresistance in the Sca1$^+$ subpopulation compared with the corresponding Sca1$^-$ cells.
TCF4 required for radioresistance of colorectal cancer cell lines
In GBM
CD133$^+$ GSC-enriched cells: more resistant to irradiation than CD133$^-$ cells (enhanced DNA repair capacities of CD133$^+$ cells)
FoxM1 promotes GBM resistance (upregulation of Rad51: critical component of the DDR process)
radioresistant GBM cells expressed high levels of WNT signaling-related genes (WISP1, FZD1, LEF1, TCF4, WNT9B, AXIN2) inhibition of WNT pathway by XAV939(WNT signaling inhibitor) sensitized GBM cells to irradiation
Cross-talk between WNT and other Signaling Pathways in GBM
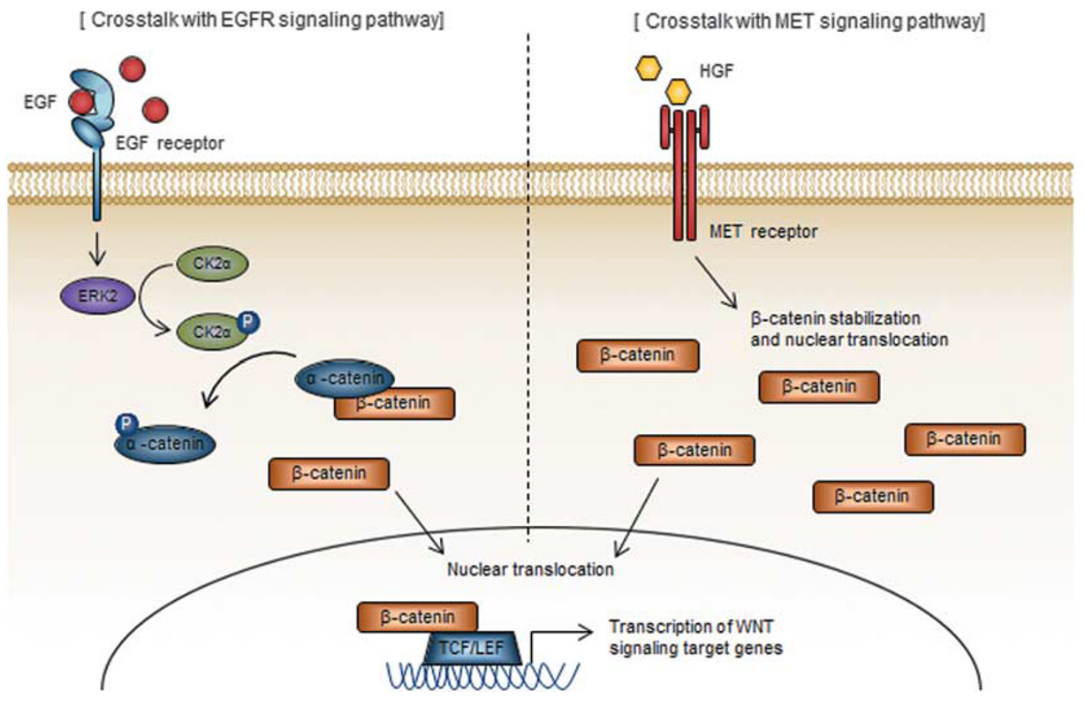
Figure 3. Cross-talk with other signaling pathways
RTKs: promote GBM survival, proliferation, invasion
90% of GBM: hyperactivation of RTK signaling(genomic amplification and/or activating mutations of RTKs)
EGFR, PDGF Rec, FGFR, MET amplification or somatic mutation ⇒ correlate with GBM subtyes
Cross-talk with EGFR Signaling Pathway
GBM 60%: EGFR amplification & hyperactivation
EGFRvIII mutation: known to contribute to cancer development
activation: induces downstream mitogenic signaling(mitogen-activated protein kinase, IP3-kinase/Akt, transducers and acivators of transcription(STAT) pathways)
β-catenin associated with Akt1, CCND1, JUN, p53, VEGFAME
mitogen-activated protein kinase, insulin, focal adhesion, adherens junction, ErbB pathwyas →proposed as β-catenin-related pathways
relationship bet. EGFR and WNT pathways
β-catenin inhibition in GBM cell lines → downregulation of EGFR, STAT3, Akt1, MMP2, MMP9, FRA01, c-MYC
TCF4 downregulation reduced Akt1 expression(binding to the Akt1 promoter)
EGF signaling is an upstream regulator of the WNT pathway
EGF-induced ERK2 upregulation → phosphorylation of CK2α → subsequent phosphorylation of α-catenin → inhibition from binding to β-catenin → shuttling of the latter into the nucleus
chronic EGF treatment resulted in downregulation of transcription of caveolin-1, E-cadherin.
loss of caveolin-1 → β-catenin transactivation
depletion of E-cadherin → prevented cell-cell connection, induced EMT
Cross-talk with MET Signaling Pathway
MET(Hepatocyte growth factor receptor): crucial roles in cancer growth, stem cell maintenance, metastasis
In GBM
expression levels of MET correspond with poor patient survival and malignancy
positive association bet. MET expression and invasiveness-related genes(MMP2, MMP9) and proto-oncogenes(c-MYC, KRAS, JUN)
MET signaling & WNT signaling connected in cancer, not fully understood
activation of MET signaling(adding HGF) induced nuclear translocation of β-catenin
inhibiting MET(by small molecules): blokade of β-catenin nuclear translocation and TCF/LEF promoter activity
⇒ possibility that MET signaling is an upstream regulator of WNT signaling
Cross-talk with Sonic Hedgehog(SHH) Signaling Pathway
SHH signaling: key pathway for cellular proliferation & tumorigenesis
SHH, WNT in medulloblastoma: prominent signaling pathways that drive the formation of distinct tumor subgroups
GLI1: physically interact with β-catenin → degradation : possibility that SHH and WNT may not be co-activated in these tumors
30%: mutations in SHH signaling components(PTCH1,, SUFU etc) → aberrant SHH signaling activation
GBM: activation of SHH pathways
blockade of SHH with Vismodegib(chemical inhibitor) → induce cell cycle arrest&apoptosis, downregulated GLI1 expression in patient-derived GBM cells
SHH signaling has a suppressive effect on WNT signaling
GLI1 binds to the sFRP1 promoter → increase mRNA expressino in GBM
Targeting the WNT Signaling Pathway in GBM
Expression levels of WNT pathway genes: associated with a poor prognosis in glioma patients
mRNA expression of β-catenin, Dvl3, cyclin D1: significantly higher in glioma
protein levels of β-catenin, TCF4, LEF1, c-MYC, n-MYC, c-JUN, cyclin D1
among them, β-catenin: significantly positive correlation with TCF4, LEF1
expression of WNT1, β-catenin, cyclin D1: associated with malignancy and clinical outcomes of GBM patients
LEF1(key effector of WNT signaling): regulate intra-tumoral heterogeneity, indicating a widespread interplay bet. WNT signaling-related TF and GBM driver pathways
WNT targeting can be an effective therapeutic approach against GBM(Table 1)
and,, WNT signaling inhibitors: therapeutic efficacy in other cancers
but few are known in GBM
WNT signaling inhibitors list that have potential for anti-GBM therapy
- non-steroidal anti-inflammatory drugs
- small-molecule chemical inhibitors
- therapeutic antibodies that target various WNT pathway components
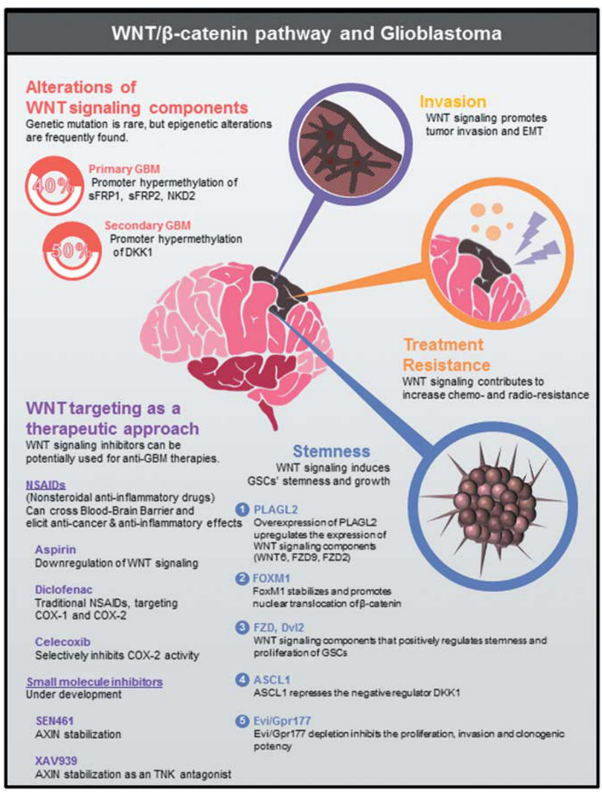
Figure 4. The multiple roles of WNT signaling in GBM
- stemness maintenance
- migration and invasion
- induction of therapeutic resistance
-
NSAIDs
inhibit activity of COX-1, COX-2
shown anti-cancer effects, cross BBB efficiently ← 오?
-
aspirin
inhibits proliferation of cancer cell lines that no not express COX-1, COX-2
downregulated WNT signaling in colorectal cancer cells
daily aspirin treatmetn for 5~ years: reduce risk of colon cancer
GBM에서: G0/G1 arrest in U87MG and A172 cells → inhibit proliferation, invasiveness (downregulation of WNT signaling)
⇒TCF/LEF promoter activity and expression of WNT signaling-target genes(c-MYC, Cyclin D1, FRA-1) decrease in GBM cell lines
-
Diclofenac, Celecoxib
reduce proliferation, colony formation, migration of glioma cells
-
- small-molecule chemical inhibitors: Table 3
-
SEN461
potenet WNT signaling inhibitor, validate molecular mechanism
prevented proteosoma degradation of AXIN → stabilization of AXIN → phosphorylated β-catenin cytoplasmic level increase → loss of total β-catenin
experimentally ok
-
XAV939
antagonist of Tankyrase(TRF-1, TNK) ← inhibiting its interaction with AXIN and regulating stability
TNK catalyses AXIN ubiquitination, XAV939 increases AXIN stabilization
but no clinical progress until now
-
-
Therapeutic Antibodies: table3
trap and neutralize WNT ligands(WNT1, 2, 5A, sFRP2), anti-FZD antibodies
effective on lung/colon/stomach/breast cancer in vitro/in vivo
how to pass through BBB? ← nanoparticle conjugation, antibody engineering are being studied
Closing Remarks
- contributes to GBM pathology at multiple levels including tumor initiation, maintenance of stem cell status, invasion, therapeutic resistance
- epigenetic silencing of negative WNT regulators, overexpression of positive regulators → aberrant activation fo WNT signaling(no genetic alteration!)
- agents are being developed

Leave a comment