GSK3 signalling in neural development
this is the re-uploaded version of my previous Naver Blog Posting at 2022-03-15
Hur, E. M., & Zhou, F. Q. (2010). GSK3 signalling in neural development. Nature Reviews Neuroscience, 11(8), 539-551. Paper Link
Abstract
GSK3(glycogen synthase kinase 3) proteins and their upstream and downstream regulators have key roles in many fundamental processes during neurodevelopment
Disruption of GSK3 signalling: adversely affects brain development, associated with several neurodevelopmental disorders
- mechanisms by which GSK3 activity is regulated in the nervous system
- provide an overview of the recent advances in the understanding of how GSK3 signalling controls neurogenesis, neuronal polarization and axon growth during brain development
GSK3: crucial node that mediates svarious cellular processes that are controlled by multiple signalling molecules(DISC1(disrupted in schizophrenia 1), PAR3(partitioning defective homologue 3), PAR6, Wnt proteins)that regulate neurodevelopment
Introduction
serine/threonine kinases, originally identified as key regulatory enz in glucose metabolism
GSK3β2: expressed specifically in the nervous system, highest levels are found during development
GSK3s as key regulators in multiple neurodevelopmental processes: including neurogenesis, neuronal migration, neuronal polarization, axon growth and guidance
Broad range of GSK3 substrates
-TF(CREB(cAMP response element-binding protein), Nfat(neuclear factors of activated T cells)family of proteins, neurogenin2, SMAD1, c-Jun, β-catenin)
important in the regulation of gene expression throughout neurodevelopment
controlling their ptn levels, DNA binding activities, nuclear localization
-MAPs(microtuble-associated proteins)
control of microtubule dynamics
cell morphogenesis regulation by reorganizing cytoskeleton(especially microtubules)⇒ control mitotic spindle reorganization, soma during neuronal migration, coordinated movement of the leading process, directed growth cone advancement during axon growth and guidance
Changes in GSK3 activity
-associated with many psychiatric, neurodegenerative diseases(Alzheimer’s disease, schizophrenia, autism spectrum disorders)
⇒GSK3 might be a common therapeutic target for different classes of psychiatric drugs
lithium(direct inhibitor, mood stabilizer)
ex> schizophrenia(DISC1, neuregulin1, frizzled3), autism(PTEN, DISC1, serotonin, TSC1-TSC2 complex, APC)
In this Review…
- overview of the involvement of GSK3 signalling in neurodevelopment with a particular emphasis on neurogenesis, neuronal polarization and axon growth
- potential crosstalk between GSK3 signalling and other pathways that are implicated in these developmental steps
Box1: Current methods used to detect GSK3 activity
-
examine Ser9, Ser21 phosphorylation
-is it the major regulatory mechanism in the CNS?
-GSK3 activity change: not always accompanied by changes in their phosphorylation status
⇒might not be used as the sole indicator of GSK3 activity
-
in vitro kinase assay
-endogeneous GSK3 canbe immuno-precipitated from cell lysates to detect its activity towards its substrates
-interaction이 transient, only small percentage of GSK3 interacts with regulator
-
examine tyrosine phosphorylation in GSK3
-this phosphorylation faciliatates the activity of GSK3 by promoting substrate accessibility.
-thought to occur through a post-translational and intramolecular autophosphorylation event, which in many cases is not dynamically regulated by external stimuli
-
examine changes in the phosphorylation status of known GSK3 substrates
-allows us to detect more physiologically relevant changes in GSK3 activity
-should consider multiple potential substrates
Regulation of GSK3 activity in the CNS
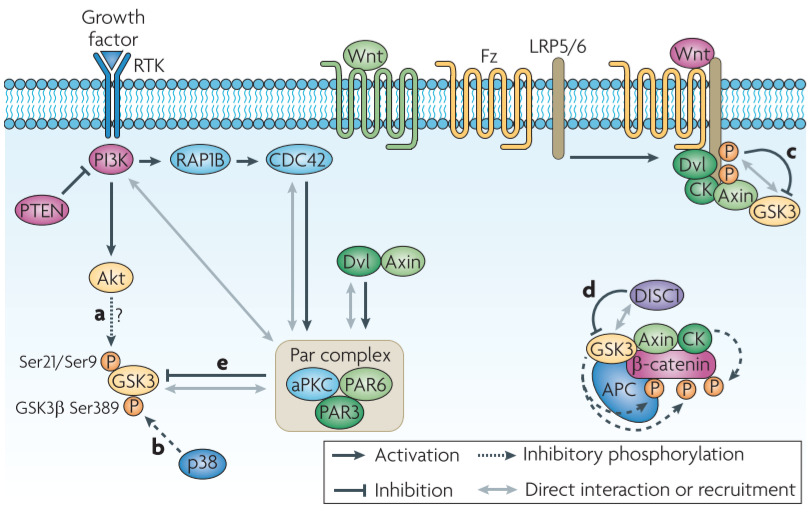
Figure 1│Proposed models of GSK3 inactivation
- high basal activity in resting cells and is inactivated by upstream regulators in response to stimuli
- activation of the PI3K(phosphatidylinositol 3-kinase) pathway downstream of RTK signalling result in inactivation fo GSK3 by serine phosphorylation
⇒Akt is the major family that mediates serine phosphorylation and the subsequent inactivation of GSK3
:x in nervous system ⇒ alternative pathways might exist in developing nervous system
- p38MAPK regulates the activity of GSK3β by inducing an inhibitory phosphorylation on Ser389
- p38MAPK-mediated phosphorylation is insufficient to provide a regulatory mechanism of GSK3 in the nervous system(∵GSK3α is also required in normal development)
- Wnt-induced dissociation of GSK3 from its substrate β-catenin leads to the stabilization and activation of β-catenin
- Wnt receptor is stimulated → GSK3 is recruited to the membrane + co-receptor LRP 5/6 directly inhibit GSK3’s activity towards β-catenin
- Regulation of GSK3 activity towards a particular substrate rather than general inhibition of its kinase activity
- DISC1 prevents GSK3 from phosphorylating β-catenin through physical interaction with GSK3
- In the Wnt and the RTK signalling pathways, GSK3 activity is also regulated by polarity proteins
- regulation of GSK3 activity by the Par complex(PAR3-PAR6-PKCζ complex) (← CDC42 at the leading edge recruits the complex) ⇒ GSK3β phosphorylation and inactivation
- Dvl and axin have been suggested to mediated GSK3 inhibition though this pathway during directed cell migration
- PAR3 directly binds PI3K and enhances its activity → explanation for the increased GSK3 phosphorylation downstream of PAR3
- regulation of GSK3 activity by the Par complex(PAR3-PAR6-PKCζ complex) (← CDC42 at the leading edge recruits the complex) ⇒ GSK3β phosphorylation and inactivation
GSK3 signalling in neurogenesis
- GSK3 signalling is essential for coordinating the proliferation and differentiation of progenitor cells during brain development
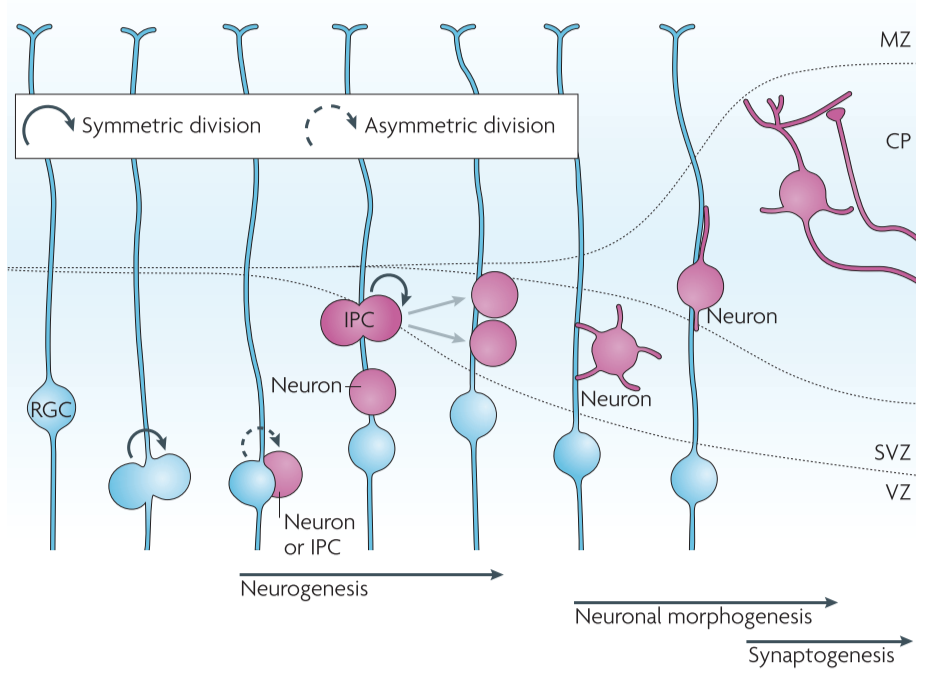
Figure 2│Neuronal development of the mammalian neocortex
GSK3 inactivation promotes progenitor proliferation
GSK3 signalling has a pivotal role in the developing nervous system
- double knockout mice
- substantially increased cortical surface area with a convoluted shape due to an over-expansion of the neural progenitor cells
- the cortex was thinner than that of control littermates, which indicated a reduction in the number of neurons
-
further analysis using various markers of progenitors, IPCs, postmitotic neurons
⇒ deletion of Gsk3 genes markedly enhanced the proliferation of progenitor cells while suppressing neuronal differentiation
⇒spatiotemporal regulation of GSK3 activity is required for an appropriate transition from the proliferative to the neurogenic phase to occur during brain development
- regulators of neural progenitor proliferation← Wnt, SHH, FGF, Notch signalling pathway etc.
- numbers of study suggest ⇒ GSK3 might function as a node molecule of multiple signalling pathways and thereby coordinate the proliferation and differentiation of enural progenitors
- DISC1 and PAR3 regulate GSK3 activity in the Wnt and the Notch pathway, respectively, and that this allows pathway-specific control of GSK3
- inhibition of GSK3 signalling, through the deletion of Gsk3s, or the overexpression of their negative regulators promotes the proliferation of progenitor cells while suppressing their differentiation into neurons
GSK3 activation promotes neuronal differentiation
- phosphorylation levels of c-Myc and β-catenin (targets of GSK3) are increased at later stages in development
- Disc1 knockdown in neural progenitors was shown to cause premature neuronal differentiation at the expense of the size of the progenitor pool
- increased GSK3 kinase activity
- pharmacological inhibition of GSK3 prevented the progenitor proliferaction defects that are induced by Disc1 knockdown
- LPA seems to increase GSK3 activity (mediated in part by the small GTPase RhoA)
- treatment of ex vivo cultured cortical hemispheres with LPA promoted terminal mitosis of neural progenitors, which led to the differentiaition of neural progenitors into neurons
- LFC(GEF of RhoA) promotes neuronal differentiation
⇒consistent with hypothesis
- extracellular cue(retinoic acid derived from forebrain meninges) is required for neuronal differentiation
- retinoic acid decreases GSK3β phosphorylation and increases GSK3 expression
⇒possibility: retinoic acid signalling leads to the elevation of GSK3 activity above basal levels to control neurogenesis
supported: PAR3 was significantly upregulated in neural progenitors in the absensce of retinoic acid
-
indirect evidence that GSK3 activation might also be regulated by extracellular cues during neuronal differentiation
: a mouse model that overexpresses GSK3 specifically in neural progenitors is needed to test this idea
GSK3 controls neurogenesis by coordinated regulation of protein degradation and microtubule reorganization
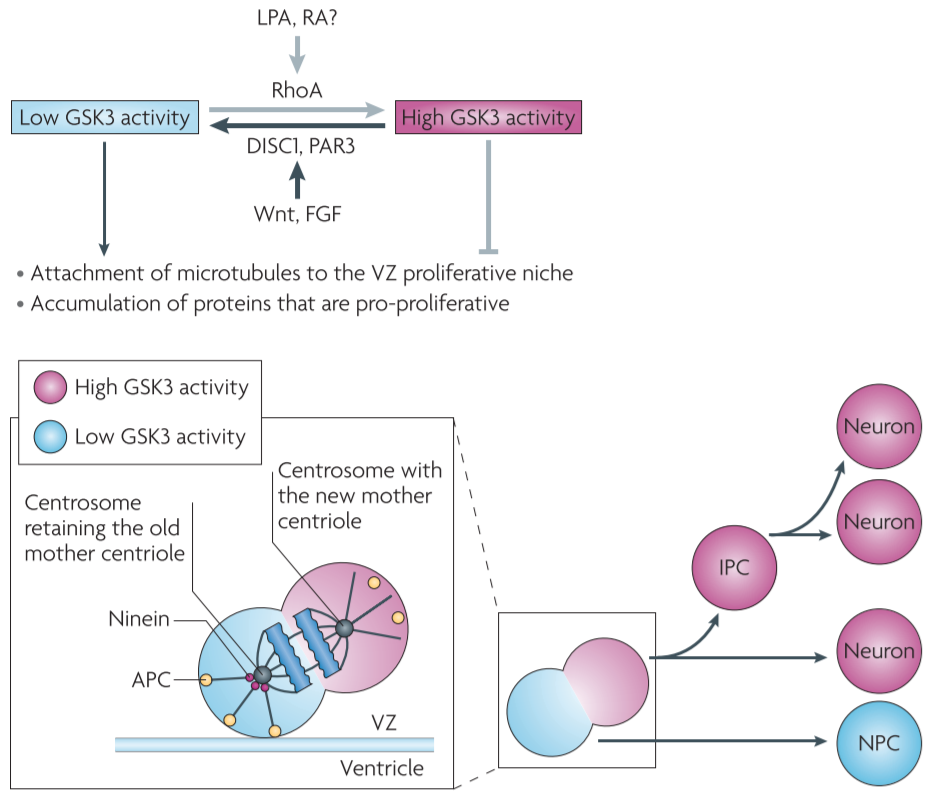
Figure 3 │ Proposed model for the role of GSK3 signalling during neurogenesis
GSK3 regulates the stability of a wide range of proteins via activation of the ubiquitin-proteasome system(UPS)
- ⇒controlling the levels of molecules that are involved in neurogenesis
-
transcriptional regulators e.g., β-catenin in the Wnt pathway, GLI in the SHH pathway, c-Myc in the FGF pathway
⇒all of which control progenitor proliferation through reglation of gene transcription
- During asymmetrical division of radial glial cells, two daughter cells have different GSK3 activity because of asymmetrical segregation of upstream GSK3 regulators(e.g., PAR3)
- during mitotic cell division GSK3-phosphorylated β-catenin, which is destined for degradation, is inherited by only one daughter cell
- The daughter cell with lower GSK3 activity will accumulate β-catenin (and perhaps other pro-proliferative proteins) and thus remains a progenitor
- The daughter cell with higher GSK3 activity will degrade these proteins through activation of the UPS and then differentiate into either a neuron or an IPC
- GSK3 could regulate other molecules that are important for controlling proliferation or differentiation.
-
during asymmetrical division of radial glial progenitors,
daughter cells that inherit the centrosome containing the new centriole migrate away from the ventricular zone(VZ) and differentiate into neurons
daughter cells that inherit the centrosome containing the original, mature centriole retain the capacity to self-renew and remain in the VZ
-
removal of ninein, mainly localized in the mature centriole, disrupts the asymmetric segregation of the centrosome and promotes neuronal differentiation
ninein is a substrate of GSK3, ninein levels are regulated by the UPS
⇒ during asymmetric division, ninein would be accumulated in the daughter cell with low GSK3 activity
ninein is a microtubule minus end-anchoring protein that is involved in the formation of astral microtubules,
- so daughter cells with ninein in their centrosome are more likely to be anchored to the apical surface of VZ and inherit a progenitor fate
- daughter cells with high GSK3 activity will have little ninein in their centrosome. This will lead to their detachment from the VZ surface and their differentiation into neurons or IPCs.
-
- MAPs(GSK3 substrates, microtubule plus end-binding proteins(microtubule plus end tracking proteins, TIPS)) control several aspects of microtubule dynamics
- When GSK3 activity is inhibited, APC binds to a microtubule plus end and there it anchors the spindle microtubules to the kinetochore or the astral microtubules to the cell cortex, both of which are important for (symmetric and asymmetric) cell division.
- APC plays a key part in asymmetric stem cell division by anchoring one daughter cell in a specific niche so that it retains self-renewal capacity
- In summary
- GSK3 signalling is emerging as a key regulator of neurogenesis
- its role in the regulation of protein stability and microtubule assembly, GSK3 signalling coordinates proliferation and differentiation.x
GSK3 signalling in neuronal polarization
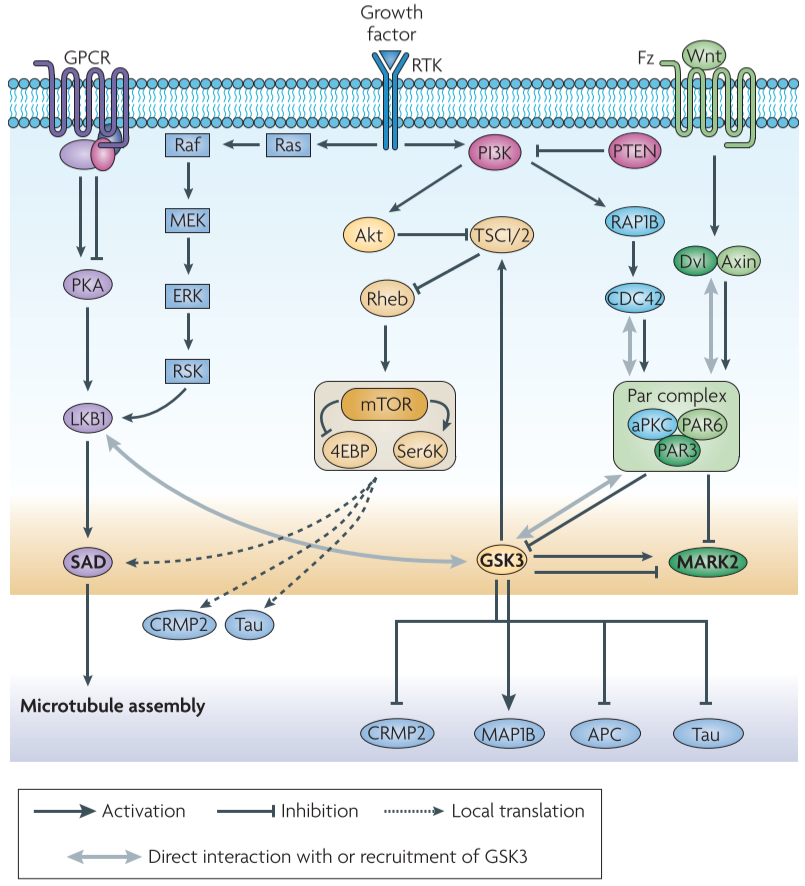
Figure 4 │ GSK3 inthe regulation of neuronal polarization
proteins that are involved in neuronal polarization: PI3K, Akt family, GSK3, small GTPases RAC1, CDC42, Par polarity complex - might act in distinct neuronal populations at specific stages of embryonic development + form a network that controls neural development in a coordinated manner
- evidence for a role of GSK3 signalling in neuronal polarization
- potential crosstalk of GSK3 signalling with other pathways that are involved in polarization
GSK3 regulates neuronal polarization by controlling microtubule dynamics
- Studies in hippocampal neurons: show that the inactive form of GSK3β is located at the tip of each neurite before polarization, but when neurons begin to polarize and one of these enurites develops into axon, phospho-GSK3β becomes concentrated at the axonal tip ⇒ maintaining local inactivation of GSK3β at the nascent axon is crucial for polarization
- global inhibition of GSK3(by small-molecule inhibitors) or knocking down of GSK3β induces the formation of multiple axons
- overexpressing GSK3β-Ser9 Ala prevents axon formation
- local inhibition of GSK3 can convert dendritic process into axons in already polarized neurons
⇒ localized inactivation of GSK3 is required for both the establishment and maintenance of neuronal polarity
- major recognization of growth cone cytoskeleton is required for neuronal polarization: loose actin network at the tip of the futue axon enables microtubules to selectively engorge into the axonal growth cone and so create the platform for subsequent axon elongation
CRMP2, APC, Tau, MAP1B→ substrates of GSK3, involved in neuronal polarization by regulating microtubule dynamics
-
binding of CRMP2 to tubulin dimers(abolished by GSK3β phosphorylation) promotes microtubule polymerization
CRMP2 is enriched in the nascent axon and overexpression of CRMP2 is sufficient to induce the formation of multiple axons
-
APC also is similar
⇒ GSK3β inactivation in the nascent axon promotes the association between APC and icrotubule plus ends and thereby stabilizes the growing ends of axonal microtubules to support axon extension
- Tau phosphorylation by GSK3β abolishes its binding to microtubules and thus impairs microtubule assembly
- GSK3 phosphorylation of MAP1B maintains microtubules in a dynamic state
GSK3 signalling and the PI3K-Akt pathway
- GSK3β acts downstream of PI3K-Akt
- PI3K-At pathway leads to the inactivation of GSK3β by inducing Ser9 phosphorylation
- Myr0Akt: induces the formation of multiple axons
- ectopic expression of PTEN precented axon formation/reversed by a GSK3 inhibitor
- knocking down Pten induced the formation of multiple axons, which was prevented by expression of GSK3β-Ser9A
- GSK3 activity is crucial for neuronal polarization, but suggest tha there might be alternative mechanisms for GSK3 inactivation
- GSK3α-Ser21Ala/GSK3β-Ser9Ala double knock-in mice develop normal polarity in vivo and in vitro → still form multipel axons when treated with various GSK3 inhibitors
- might be alternative pathway downstream of Akt for the regulation of polarization
GSK3 signalling and the PAR3-PAR6-PKCζ polarity pathway
PAR3-PAR6-PKCζ complex is required for axon-dendrite specification of hippocampal neurons
- PAR3, PAR6 become selectively enriched in the nascent axon and in the developing growth cone ← negative regulators of GSK3
- suggest: there is feedback regulation between PAR3 and GSK3
- in hippocampal neurons Dvl becomes enriched at the tip of the axon in response to Wnt5a and induces axon formation through activationof PKCζ in complex
- Dvl overexpression leads to the production of multiple axons but does not affect GSK3β-Ser9 phosphorylation, and Dvl is still able to induce multiple axon formation in the presence of GSK3β-Ser9Ala.
- In this model..
- activation of PKCζ in response to Wnt5a-Dvl leads to the inhibitory phosphorylation of MARK2, which then causes dephosphorylation of MAPs, such as Tau
- knocking down Mark2 decreased Tau phosphorylation and induced the formation of multiple axons, whereas ectopic expression of MARK2 increased Tau phosphorylation and blocked axon growth
- however, based on the remarkable similarity of the factors(Wnt5a-Dvl-PKCζ) that are involved in neuronal polarization and cell migration, it could be suggested that GSK3 inactivation has a role downstream fo Wnt-Dvl-PKCζ to control neuronal polarization as well.
- inactivation of MARK2 and GSK3β at the axonal tip together reduces Tau phosphorylation downstream f the PAR3-PAR6-PKCζ complex
- GSK3β can directly activate as well as inhibit MARK2 through phosphorylation
⇒ exact roles of MARK2 and GSK3β, and their interplay during neuronal polarization remain to be fully defined
GSK3 signalling and the LKB1-SAD pathway
SAD kinases are required for neural development in vivo
SAD kinases play a pivotal part in neuronal polarization
LKB1 and SAD kinases are required for neuronal polarization in vivo
XEEK1(Xenopus orthologue of LKB1) regulates GSK3 activity and physically associates with GSK3β and aPKC in vivo
GSK3 signalling and the TSC-mTOR pathway
mutations in TSC1, TSC2: cause tuberous sclerosis(tumour predisposition and neurological abnormalities)
TSC1 and TSC2 form a heterodimer that negatively regulates mTOR signalling
On activation of the PI3K-Akt pathway, Akt induces inhibitory phosphorylation of TSC2, by which its GAT activity towards Rheb is reduced, subsequently leading to the activation of mTOR kinase.
mTOR then phosphorylates translational regulators(S6K, 4EBP)→induces protein synthesis
- inactivation of the TSC1-TSC2 complex and subsequent mTOR activation in a single neurite regultaes axon formation by inducing the translation of polarity proteins such as SAD kinases.
- phosphorylated forms of S6K and 4EBP are enriched in the axon during polarization, which suggests local activation of mTOR signalling
- with the elevation of SAD kinase activity by LKB1 that is mentioned above, the control SAD protein levels by TSC-mTOR signalling might have a role in axon specification
(Akt phosphorylation of TSC2 has been suggested as an alternative mechanism to convey PI3K-Akt signalling)
direct interaction between TSC-mTOR signalling and GSK3 has been reported in the regulation of cell growth.
suppression of GSK3 activity in the axonal growth cone has a role in the activation of local translational machinery that is used in the synthesis of key proteins for axonogenesis
A role of GSK3 activation in dendrites?
Does active GSK3 in dendrites play a part in polarization?
⇒local protein degradation through activation of the UPS is a possible mechanism for the control of asymmetrical accumulation of polarity proteins.(∵Inhibition of the UPS with MG132 or lactacystin before or after the establishment of neuronal polarity leads to the formtion of multiple axons)⇒ UPS activation is required for the establishment and maintenance of neuronal polarity
- Akt, PAR3, aPKC are ubiquitylated at early stages of polarization before they accumulate at axonal tips
- UPS inhibition restores the presence of Akt in all neurites and this restoration is accompanied y multiple axon formation ⇒ Akt is a target of the UPS for local degradation in dendrites
- GSK3β regulates proteolytic degradation of a much broader range of proteins than was previously appreciated
GSK3 signalling in axon outgrowth
coordinated regulation of local axon assembly at the growth cone and of gene transcription in the neuronal soma is required for efficient axon growth.
Differential regulation of GSK3 substrates to control local axon assembly
inhibition of GSK3 can both enhance and prevent axon growth depending on the substrates that are involved
many substrates of GSK3s require phosphorylation by a distinct kinase - an event known as priming - before they can be phosphorylated by GSK3s (primed substrates), although GSK3s can also phosphorylate some substrates without priming(unprimed substrates)
-
APC, CRMP2: primed substrates → GSK3 phosphorylation abrogates their microtubule binding affinity
⇒dephosphorylated forms of CRMP2 and APC are enriched in the growth cone
⇒promotes axon formation and mediates neurotrophin-induced axon growth
-
MAP1B: unprimed substrate → phosphorylation of MAP1B renders microtubules more dynamic, so that they can efficiently probe the intracellular space and respond to extracellular signals, and these activities are essential for axon growth
∴GSK3 activity should be precisely controlled so that its activity towards one subset of substrates is specifically blocked while its activity towards others is preserved
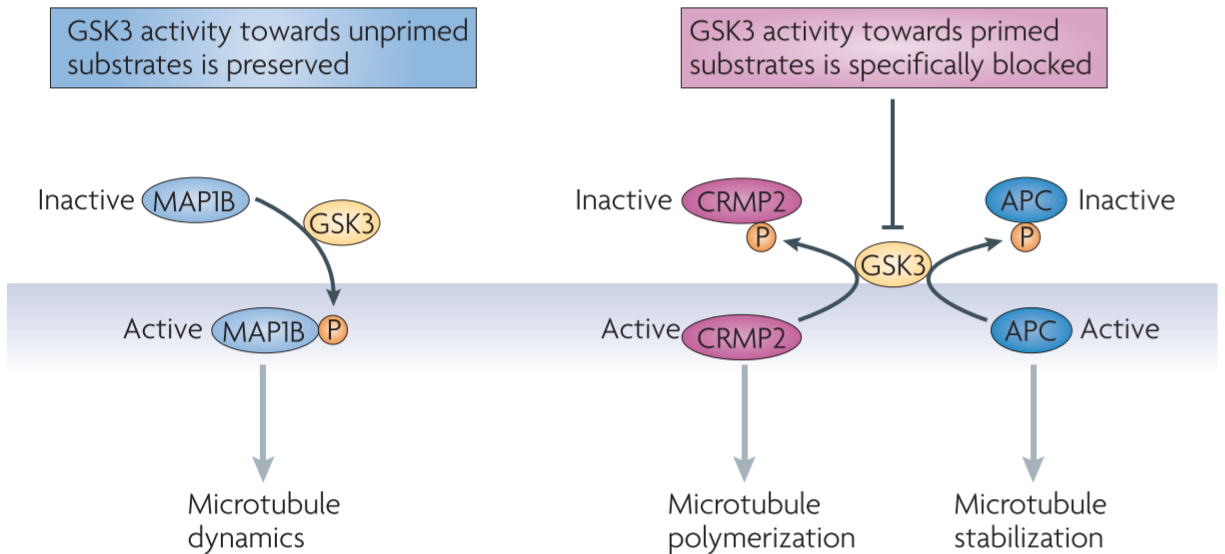
Figure 5 │ Differential regulation of GSK3 substrates during axon growth
Transcriptional control of axon outgrowth by GSK3 signalling
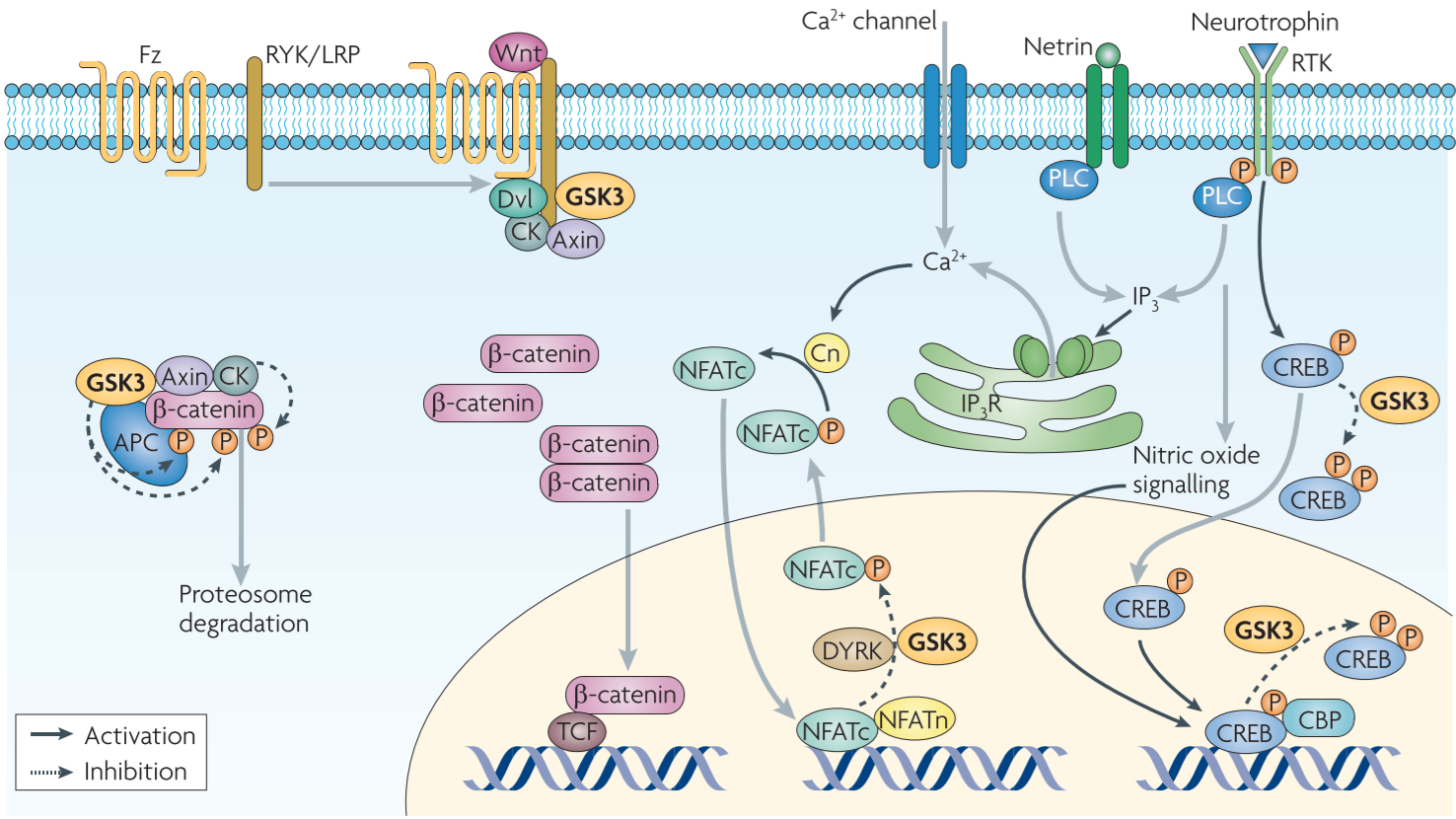
Figure 6 │ Potential roles of GSK3 in the transcriptional regulation of axon growth
GSK3: transcriptional control of axon growth through regulation of β-catenin and the NFAT family of TFs
Wnt pathway: Wnt3a induces axon growth from developing sensory neurons through accumulation of β-catenin and subsequent activation of TCF4
GSK3 could also play a part in the transcriptional control of neurotrophin-induced axon growth by phosphorylating CREB and NFAT proteins.
Neurotrophins (and other stimuli) lead to the activation of CREB kinases, which phosphorylate CREB on the transcriptional regulatory site Ser133, thereby recruiting transcriptional co-activator CBP and promoting the assembly of basal transcriptional complex
GSK3 and nitric oxide signalling work in concert to control the duration and intensity of CREB-dependent transcription
Neurotrophins and neutrins also induce transcription of genes that are essential for axon growth by triggering $Ca^{2+}$/calcineurin-dependent nuclear translocation of NFAT family of proteins
NFAT phosphorylation of GSK3 inhibits its DNA-binding acitivity and is required for nuclear export ⇒ GSK3 is likely to have a role in NFAT-mediated gene transcription during axon growth
Concluding remarks and future directions
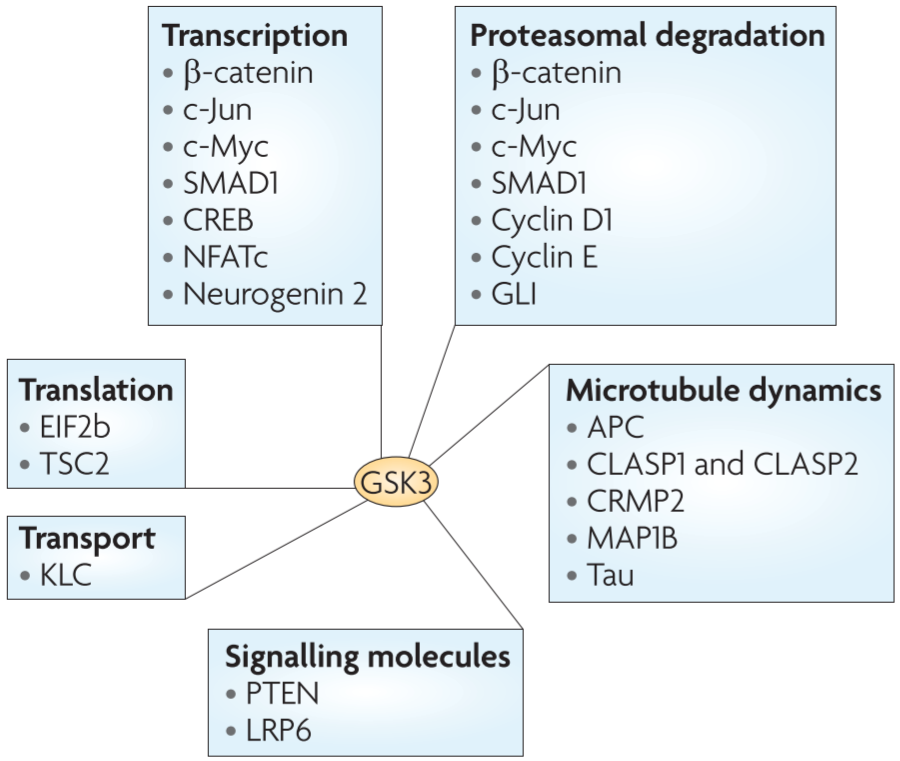
GSK3 is a kinase with a large number of substrates ⇒ could affect a broad range of cellular activities / GSK3 in many fundamental processes during neural development, and a number of neurological disorders are associated with deficits in GSK3 signalling
important to understand GSK3 signalling
- a re-evaluation of the involvement of GSK3 using more reliable methods to detect GSK3 activation or inactivation
- identification of additional in vivo GSK3 substrates
in combination with the signalling that directly regulates GSK3 activity, signalling that regulates the phosphorylation status of its substrates allows GSK3 signalling to achieve further specificity
GSK3 activation ↔ inactivation

Leave a comment