Chemiosmotic coupling in oxidative and photosynthetic phosphorylation
this is the re-uploaded version of my previous Naver Blog Posting at 2022-03-31
Mitchell, P. (2011). Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1807(12), 1507-1538.Chicago Paper Link
1. Introduction
1.1. The basic question of the coupling mechanism
o/r(oxido-reduction) particles(2H, H-, 2e-)
h/d(hydro-dehydration) particles(energy-rich)
how are the flows in the o/r and h/d pathways coupled to each other?
⇒coupling may occur through a chemiosmotic type of mechanism: that doesn’t require chemical intermediates common to o/r and phosphorylation
orthodox chemical coupling hypothesis vs chemiosmotic coupling hypothesis
1.2. Outline of the chemical hypothesis
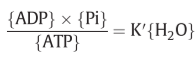
ATP hydrolysis energy = H2O 5microM→55M (10000cal/mole or 0.42eV per molecule of water) at [Pi]=10mM, pH=7
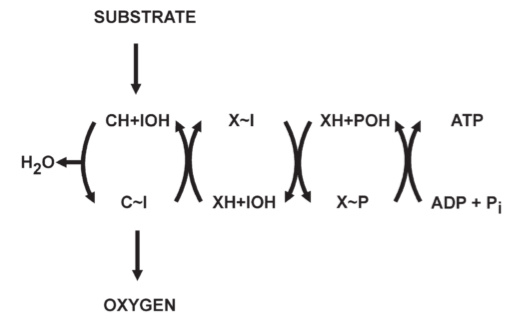
Fig 1. Outline of the chemical coupling hypothesis: one phosphorylation site in oxidative phosphorylation, C:는 carrier
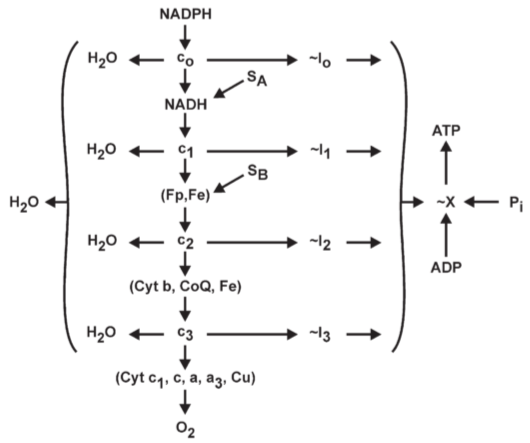
Fig 2. Outline of the chemical coupling hypothesis: four phosphorylation site in oxidative phosphorylation
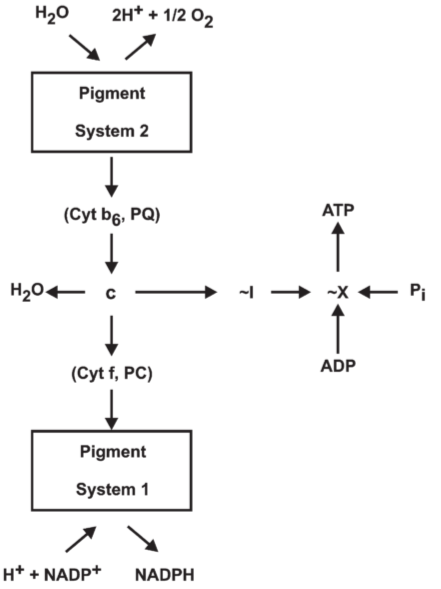
Fig 3. Outline of the chemical hypothesis: non-cyclic photophosphorylation
1.3. The question of the existence of C~I intermediates
- no generally accepted coupling intermediate common to the o/r and h/d pathways has been isolated or characterised
- no unequivocal evidence for the existence of coupling intermediates of this kind has been obtained
1.4. Objects of the chemiosmotic hypothesis
- provide a simple rationale for the organisation of the cmponents of the o/r and h/d pathways in the lipid membrane systems of mitochondria and chloroplasts
- formulate a type of coupling that would require no intermediates
- acknowledge the elusive character of the C~I intermediates by admitting that they may not exist
2. Derivation of the chemiosmotic postulates
2.1. The anisotropic o/r system
prev research: cytochrome system were anisotropically organised across a membrane
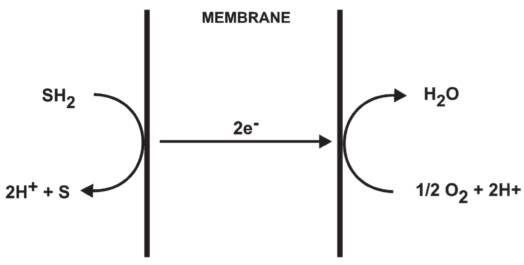
Fig 1. Electron translocating oxido-reduction system
fails to eliminate the necessity of the chemical coupling step between electron transport and phosphorylation
(synthesis of ATP would presumably have to be chemically coupled to the supposed second electron transport system)
2.2. The anisotropic h/d system
2.3. An electric component of the protonmotive force
more primitive form of the chemiosmotic coupling hypothesis
If the pH difference is above a certain level → electron transport would reverse ATP hydrolysis, and ATP hydrolysis would exert a back pressure on electron transport
but pH difference appears to be too large to work simply
→there need be onyl a relatively small pH difference
2.4. Exchange-diffusion systems
another problem: membrane potential that would now be required to reverse the ATPase reaction would cause the ions of opposite sign of charge to the internal aqueous phase to leak in through the coupling membrane
→ion leakage would have to be balanced
therefore: coupling membrane contains exchange diffusion systems: strictly couple the exchange of anions against OH- ions and of cations against H+ ions
2.5. Sophistication of the anisotropic o/r system: The o/r loop
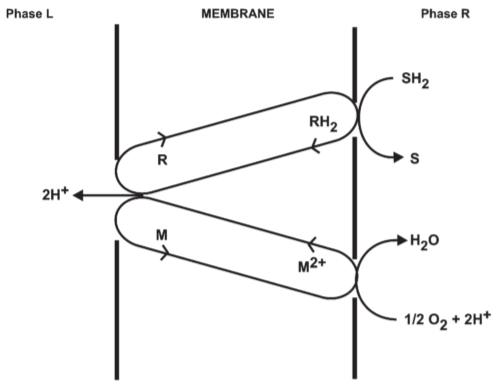
Fig 2. Proton translocating oxido-reduction loop composed of a hydrogen carrier(R/RH2) and an electron carrier(M/M2+)
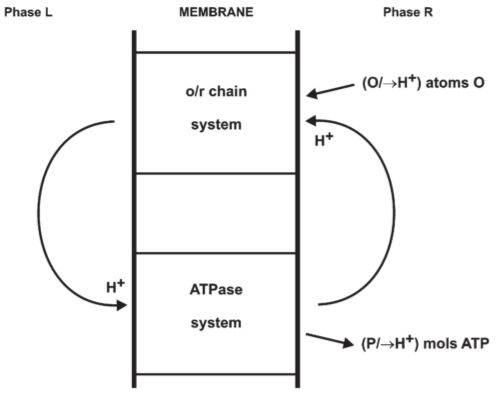
Fig 3. Stoichiometry of chemiosmotic coupling
2.6. The coupling proton circuit and P/2e values
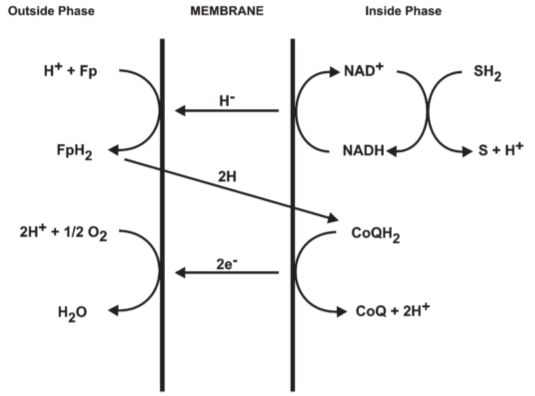
Fig 4. Mitchell’s original suggestion: incomplete
2.7. Polarity of the protonmotive force relative to the coupling membrane system
pH directionality of mitochondria and chloroplast
2.8. Summary of the basic postulates
- mt/chl membrane-located ATPase systems: hydro-dehydration systems with terminal specificities for water and ATP; their normal function is to couple reversibly the translocation of protons across the membrane
- oxido-reduction chain systems: catalyse the flow of reducing equivalents: couple reversibly the translocation of protons across the membrane to the flow of reducing equivalents during oxido-reduction
- exchange-diffusion carrier systems: reulate the pH and osmotic differential across the membrane(without collapse of the membrane potential)
- 1, 2, 3 systems: located in a specialised coupling membrane which has a low permeability to protons and to anions and cations generally
3. The proton translocating ATPase system
3.1. Proton translocating hydro-dehydration reactions
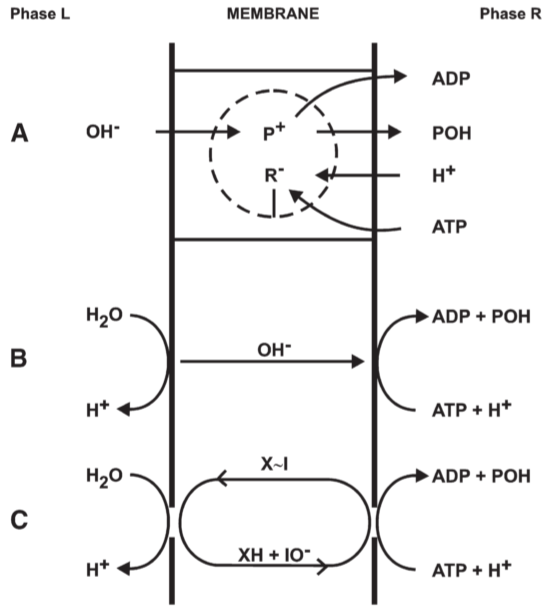
Fig 5. Proton translocating reversible ATPase system of type I: connected by OH- translocation
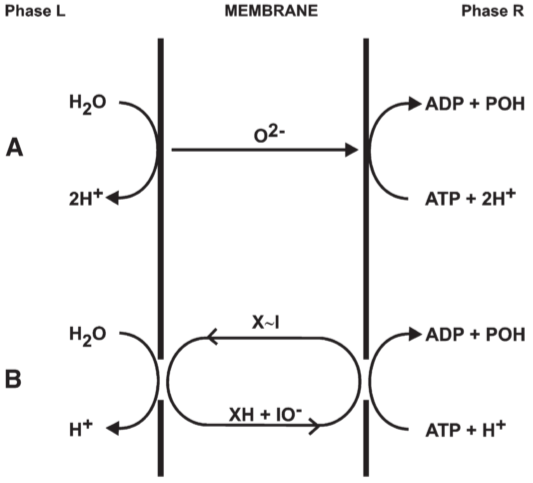
Fig 6. Proton translocating reversible ATPase system of type II: connected by O2- translocation
3.2. ATPase I and ATPase II: Mechanism and poise of equilibrium
focusing on ATPase II..
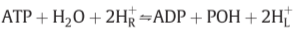
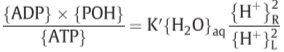
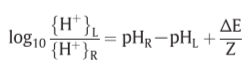
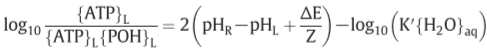
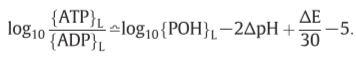
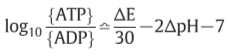
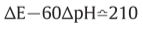
-
Assumptions
Z=2303RT/F(F: Faraday, R: gas constant)=60mV(300K)
K’=5
[POH]=0.01M
for becoming [ATP]=[ADP]…?
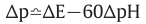
:P.M.F.(proton motive force)
ATPase II: ΔpH=-3.5 or ΔE=210mV => establish
ATPase I: ΔpH=-7 or ΔE=420mV => establish
3.3. Further discussion of ATPase II
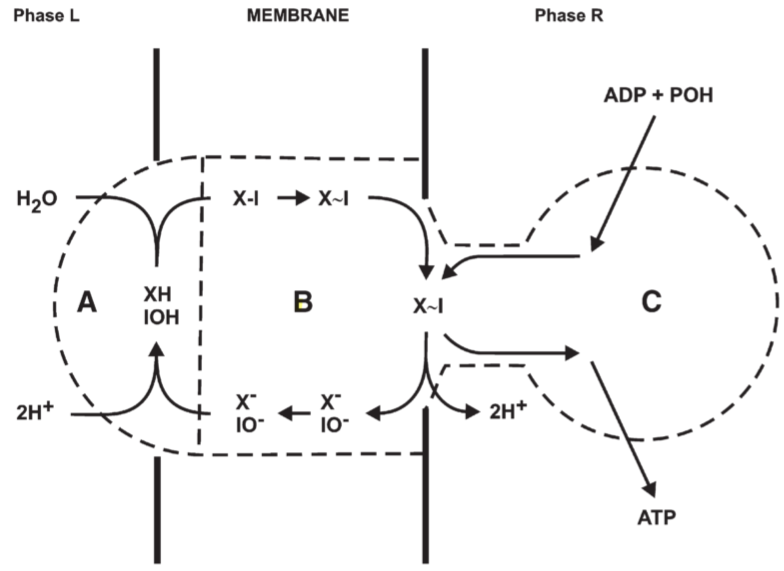
Fig 7. mechanism suggested for the reversible anisotropic ATPase II reaction. A: X-I hydrolase, B: X-I translocase, C: X-I synthetase
- The system must represent the correct stoichiometry, so that the overall reaction would naturally poise at the known thermodynamic equilibrium
- The intermediates or transition states through which the components of the system would pass as equilibrium was approached should all occur in sufficient concentration
- Phase L:
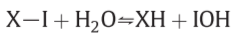
- Phase R:
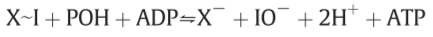
⇒X~I X~I Hydrolysis Gibbs free energy -10000cal or hydrolysis constant about 10^5M
Fig7 mechanism: require the effective pK values of the acidic groups XH and IOH to be a littele below the normal pH or phase L, or in the region of pH6
3.4. Practical reversal of the ATPase reaction
3.4.1. Spatially isotropic conditions
- would not have a close counterpart in an isotropic system
- thermodynamic considerations: ATP-synthesising function of the ATPase might possibly be reproducible under spatially isotropic conditions.
3.4.2. Spatially anisotropic conditions
- ATP and water should be catalysed by the ATPase at a fairly central poise of the ATP/(ADP+Pi) couple when balanced by the appropriate P.M.F. independently of the source of the P.M.F.
- the driving force on ATP synthesis is the P.M.F. and not the pH differential
- a P.M.F. of some 210mV should be required to drive ATP synthesis at a central poise of the ATP/ADP couple via ATPase II in the presence of 10mM Pi
- the amount of ATP synthesised via ATPase II should be given by the total number of protons passing across the coupling membrane at a P.M.F. sufficient to drive ATP synthesis at the existing ATP/ADP poise, multiplied by the proportion of the total proton flux which passes specifically through the ATPase system
- the synthesis of ATP, driven by a pH differential, may be stimulated by specific reagents, (gramicidin, valinomycin) that can collapse the membrane potential without collapsing the pH differential
3.5. Coupling factors and the ATPase system
F$_1$: molecular weight 280000, Mg$^{2+}$-dependent ATPase activity
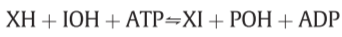
when the ATPase activity of F$_1$ is expressed the elements of H$_2$O artificially take the place of XH and IOH
characteristic spherical units that appear to be attached by stalks to the inner side of the cristae membrane of whole mitochondria, and to the outer side of the cristae membrane of sonically disintegrated mitochondria
- oligomycin insensitive ATPase reaciton catalysed by isolated F$_1$
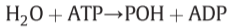
- oligomycin-sensitive ATPase of the complete ATPase II system

chloroplasts: little information, but fundamentally similar to the mitochondrial ATPase
4. The proton translocating oxido-reduction chain
4.1. The o/r loop: Further development
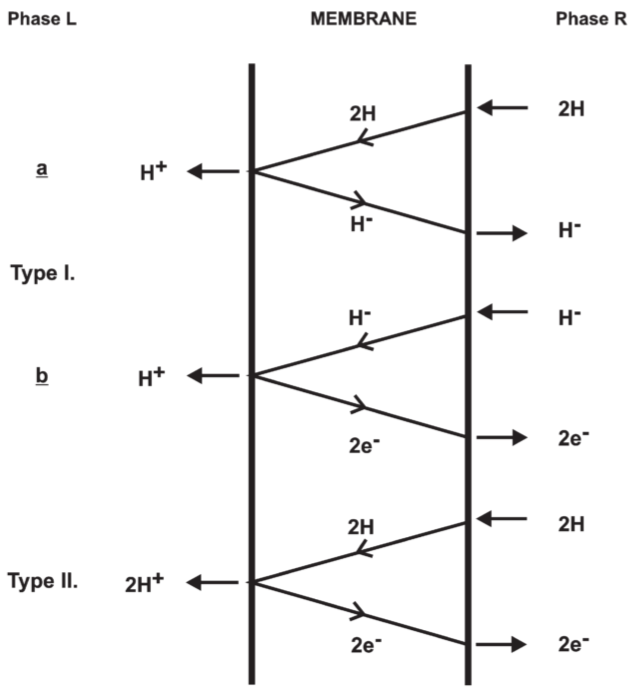
Fig 8. Possible proton translocating oxido-reduction loops of type I and type II.
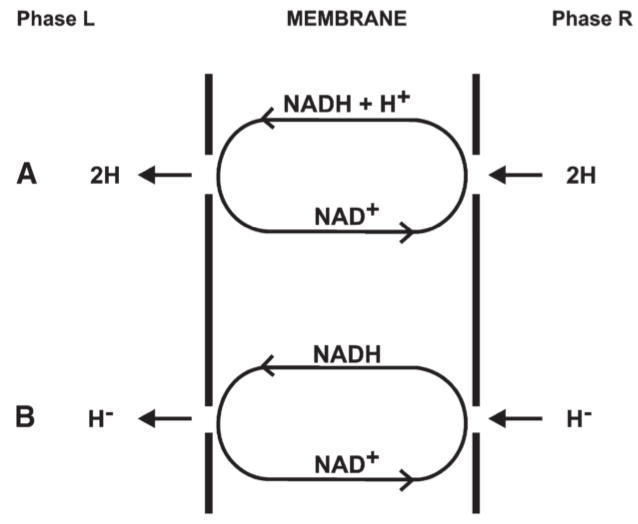
Fig 9. Effect of translocational specificity upon the currency of oxido-reduction
when { }means electrochemical activity
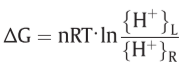
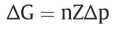
Z=2303RT/F , Δp=P.M.F.
o/r potential span of ΔE’
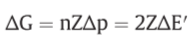
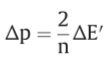
f: net driving force on the oxido-reduction reaction
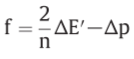
4.2. Mitochondrial o/r loops
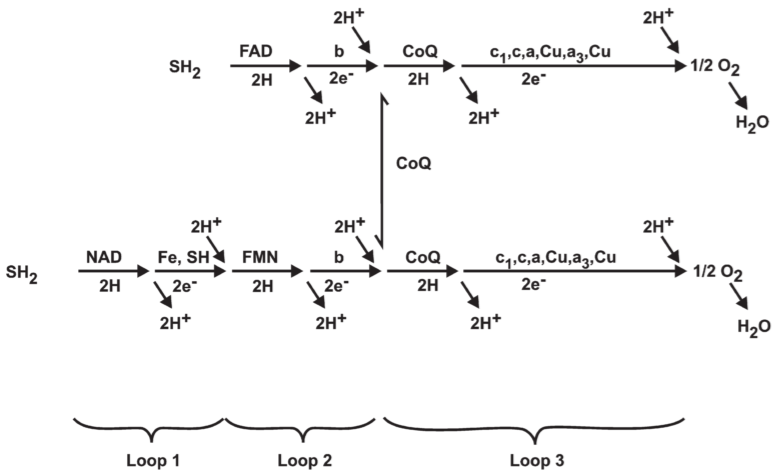
Fig 10. Suggested respiratory chains for oxidation of substrates(SH2) linked through
4.3. “Energy coupling” with o/r loops
relationship between the force (f) on the oxido-reduction process across a given o/r loop, the o/r potential span(ΔE’) across the loop and the P.M.F(Δp) across the membrane
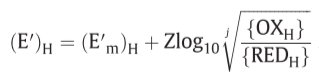
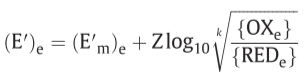
E’: midpoint potential, j/k: respective numbers of electron equivalents donated per mole of the hydrogen carrier and accepted per mole of the electron carrier
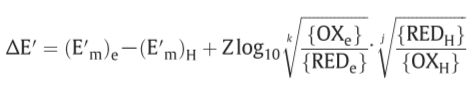
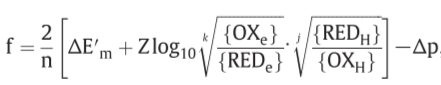
($ΔE’_m = (E’_m )_e - (E’_m ) _H$)
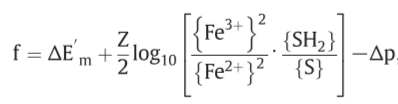
(hydrogen carrier: S/SH$_2$, electron carrier: Fe$^{3+}$/Fe$^{2+}$)
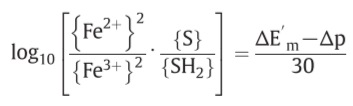
4.4. Possible composition of Loops 1, 2, and 3 in mitochondria
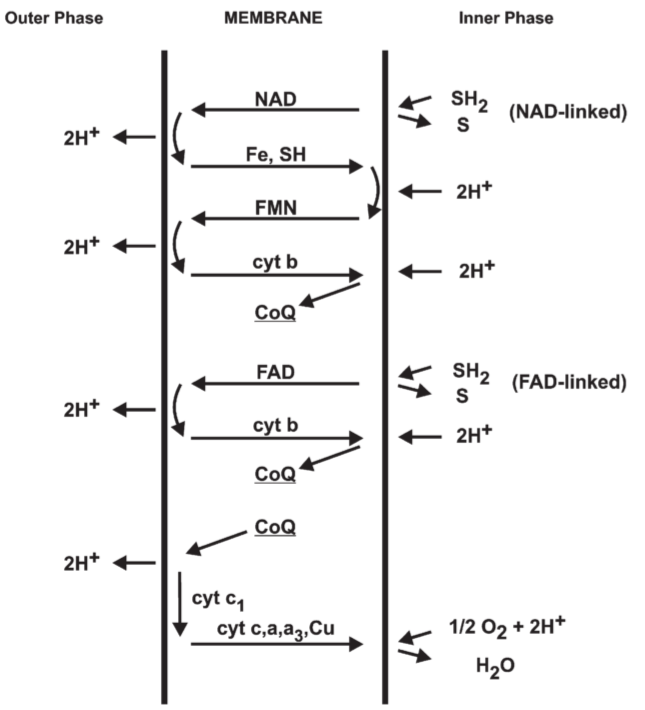
Fig 11. Suggested folding of the proton translocating respiratory chain for oxidation of NAD-linked and FAD-linked substrates in mitochondria.
4.5. Survey of mitochondrial electron and hydrogen transfer systems
4.5.1. Loop 2 an Loop 3 regions
4.5.2. Revelant disintegration and reintegration studies
4.5.3. Loop 1 region
NADH-CoQ reductase ← NADH-dehydrogenase is the terminal part
4.5.4. Phosphorylating and non-phosphorylating respiratory chains
4.6. Loop 0: The energy-linked pyridine nucleotide transhydrogenase
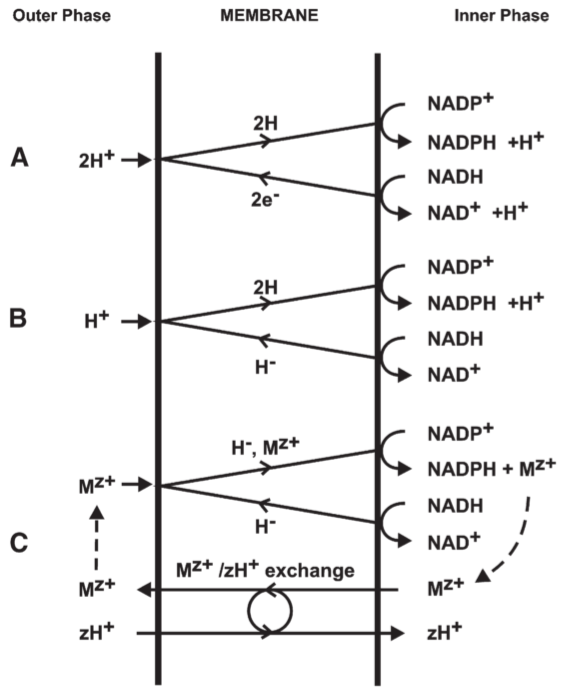
Fig 12. Possible types of proton translocating oxido-reduction loop for the transhydrogenase or Loop 0 region of the respiratory chain of mitochondria
- The reduction of NADP by NADH resembles reversed electron and hydrogen transfer nearer the oxygen end of the respiratory chain in that it is coupled to ATP hydrolysis or to oxido-reduction elsewhere in the respiratory chain system
4.7. Photon-energised electron and hydrogen transfer in photophosphorylation
not the pH differential, but the P.M.F. which poises oxido-reduction against ATP hydrolysis.
(pH differential: only one component of the P.M.F.)
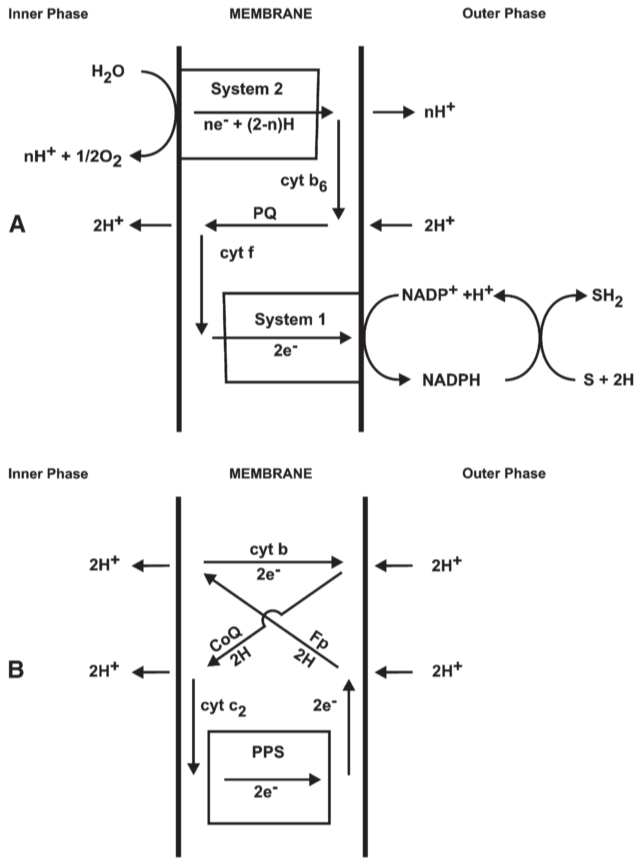
Fig 13. A possible proton translocating oxido-reduction system for noncyclic photophosphorylation in chloroplasts.
4.8. Effects of alternation between hydrogen and electron carriers
Keilin-Hartree type heart mitochondrial preparations catalyse a transfer of tritium from succinate to NAD during reversed electron and hydrogen transfer should probably be attributed to exchange via malate dehydrogenase and not to transfer through the respiratory chain.
5. The coupling membrane
: cristae and grana membrane of mitochondria and chloroplasts
plasma membrane and chromatophore membrane of bacteria
5.1. Quantitative significance of charge displacement through the coupling membrane
owing to the large interfacial area, the electric displacement between the two aqueous phases does, in fact, correspond to a significant displacement of chemical substance from one phase to the other
5.2. Energy storage capaity of coupling membrane system
greater part of the P.M.F. may be a membrane potential under normal conitions, and that only after damaging the chloroplasts does the pH differential represent the main component of the P.M.F
5.3. Membrane permeability and the conservation of the electric displacement
6. The proton circuit network
6.1. Exchange diffusion systems
diffusion of ions other than protons down the electrical gradient across the coupling membrane, and their accumulation in osmotically disruptive concentrations in the internal phase, must be conterbalanced by specific extrusion in exchange for protons or hydroxyl ions.
specific translocations sytems (in rat liver mitochondria) for the entry of inorganic phosphate, citrate, malate, and other Krebs cycle acids.
protons exchange with sodium ions across the coupling membrane of rat liver mitochondria
6.2. Coupling between proton, anion, and cation cirtuits
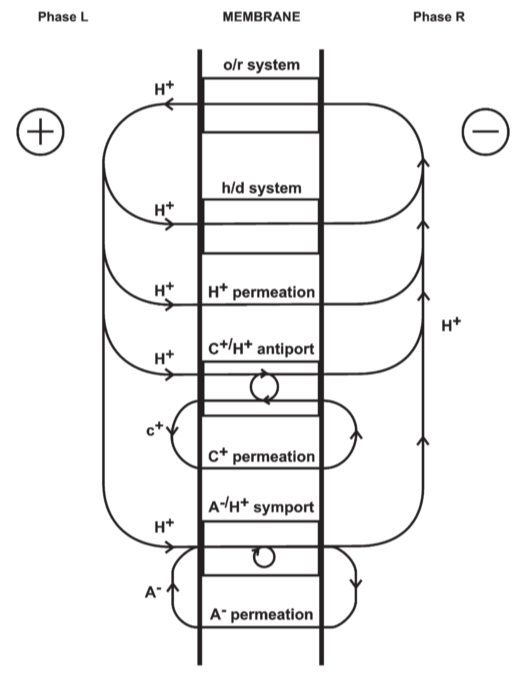
Fig 14. Coupling through proton circuits.
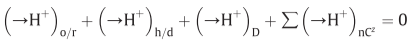
D: diffusion, fourth term: effective proton translocation coupled to the translocation of cations or anions(C) of valency +z or -z respectively
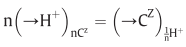
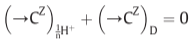
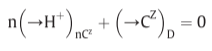
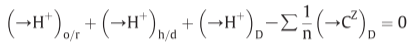
(in the steady state)
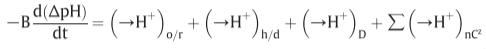
(in the non-steady state)
B: quantity of protons passing through the membrane
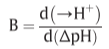
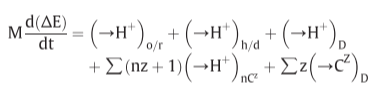
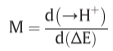
7. The integral process of proton transport phosphorylation
7.1. Respiratory control
7.1.1. Formation of the coupling membrane potential
7.1.2. The mechanism of action of oligomycin
7.1.3. Relationship between kinetics of oxido-reduction and proton translocation
7.2. The mechanism of uncoupling
7.2.1. Cation conductors
K$^+$ uptake, Ca$^{2+}$ uptake
7.2.2. Divalent cations
Ca$^{2+}$, Sr$^{2+}$, Mn${}$$^{2+}$ exhibit a special type of stoichiometric uncoupling effect
7.2.3. Dinitrophenol and other proton conductors
2.4-dinitrophenol(DNP): react with one or more of the hypothetical energy-rich intermediates of oxidative phosphorylation
⇒cause the entry of water into the ADP+P$_i$ dehydrating system
carbonyl cyanide p-trifluoromethoxyphenylhydrazone(CFCCP)
(1) DNP and CFCCP are specific proton conductors
(2) The state of the mitochondrial suspension after the outward translocation of protons through the o/r or h/d system differs in a subtle way from the state of suspension after the external pH has been brought to the same value by the addition of HCl
7.2.4. Uncoupling of photophosphorylation
7.2.5. Other uncouplers
7.2.6. Respiratory inhibition by uncouplers
7.2.7. ATPase activity and the o/r state of the carriers
7.2.8. Enzyme inhibition by lipid-soluble reagents generally
7.3. Site specific reagents
7.3.1. Oxidative phosphorylation
7.3.2. Photophosphorylation
7.4. Reversal of electron and proton transport
7.5. Osmotic pressure and water flow
ATPase II system would not actually transport protons inwards during ATP synthesis in mitochondria, but would transport O$^{2-}$outwards.
→equivalent to the translocation of 2H$^+$ inwards and H$_2$O outwards.
⇒reversible o/r linked and h/d linked swelling and shrinkage changes of mitochondria, chloroplasts, and bacteria can probably be ascribed mainly to the flow of water
8. The sidedness of the chemiosmotic system
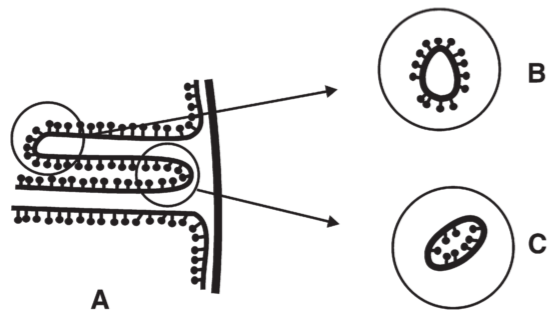
Fig 15. Diagram showing relative sidedness of mitochondrial cristae membrane. A: 정상, B: sonic disintegration, C: digitonin treatment
cytochrome c is present on the surface of the cristae membrane
components with which it reacts (cytochrome a and cytochrome c$_1$) must also be accessible to cytochrome c from outside
9. Summary
- chemiosmotic hypothesis
explain the coupling between oxido-reduction and phosphorylation without assuming the existence of chemical intermediates
four postulates
- the proton translocating reversible ATPase system
- The proton translocating oxido-reduction chain
- The exchange diffusion systems, coupling proton translocation to that of anions and cations
- The ion-impermeable coupling membrane, in which systems 1, 2, and 3 reside.
- chloroplast/mitochondria: ATPase II
- o/r loops: consisting of one hydrogen and one electron carrier
- proton motive force(P.M.F.): pH differential+membrane potential
- coupling between oxido-reduction and phosphorylation can be described by a circulating proton current connecting the ATPase and o/r systems at a P.M.F. of some 250mV

Leave a comment